 |
Vol.3, No.2
The Chemical Educator © 1998 Springer-Verlag New York, Inc. |
ISSN 1430-4171
http://journals.springer-ny.com/chedr S 1430-4171(97)02198-9 |
 |
Vol.3, No.2
The Chemical Educator © 1998 Springer-Verlag New York, Inc. |
ISSN 1430-4171
http://journals.springer-ny.com/chedr S 1430-4171(97)02198-9 |
Spectrophotometry CD-ROM for Macintosh or Microsoft Windows, published by ScienceMedia, Inc. (Copyright 1993-1997). System Requirements: Macintosh; System 7.5 or higher, 68040 or PowerPC processor, 6 MB free RAM, 2.6 MB free disk space, 4x or faster CD-ROM drive recommended. PC; Pentium-based PC or compatible, Windows 95 or Windows NT 4.0 or higher, 16 MB RAM, 2.3 MB free disk space, 4x or faster CD-ROM drive recommended.
Buttons to the right of the EXIT button
provide access to: a science dictionary (A–Z button) whose words
are highlighted in yellow throughout the program; the Table of Contents,
which lists each major section (module) of the program and its subsections;
a module map, which returns the user to this introductory screen and enables
subsequent movement to the start of any module or subsection in the program;
the help file, which explains these buttons and other components of the
screen (Figure 2); a return arrow, which takes the user back to the latest
branch point in the presentation; a single-frame-back arrow; and a single-frame-forward
arrow.
Screen layout throughout is clear, colorful, and interesting. Responses to all buttons were reliable; access speed was dependent on the performance of the CD-ROM drive.
The Introduction module provides a brief overview of the source of color, the absorption spectrum (of chlorophyll A), and the uses of such spectrophotometry.
The Background module presents information in two subsections: The Nature of Light and The Electromagnetic Spectrum. The treatment of the nature of light is rather superficial and would not suffice unless the user already had a good understanding of both the wave and the particle models that explain its behavior. The inclusion of a surfing video may be interesting, but it does little in this context to explain the wave phenomenon. This section ends with the presentation of a standard diagram of the electromagnetic spectrum, with the visible region enlarged to display its colors.
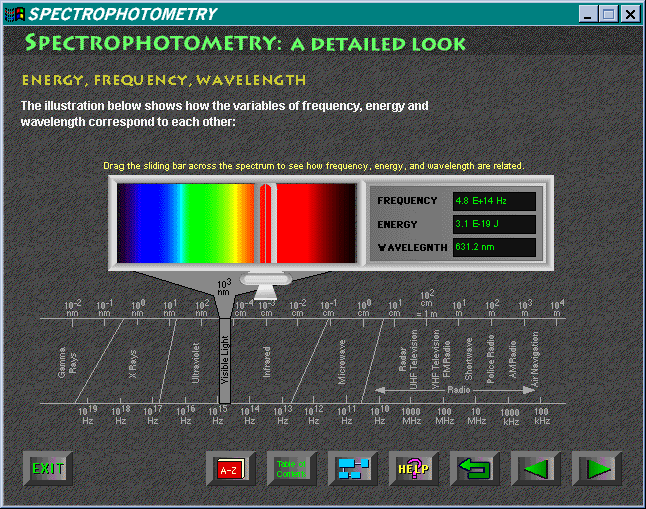
Spectroscopy: A Detailed Look is the next
module. This section introduces the relationship between the frequency
of light and its energy, and develops the formula E = hn.
A most instructive interactive feature is the user-controlled slide bar;
when this is moved along the representation of the visible spectrum, one
sees simultaneous readouts of frequency, wavelength, and energy (Figure
3).
The screen states: "Visible light appears to us as a white light, but is composed of a spectrum of colors familiar to us from rainbows." This seems rather late in the sequence to introduce this idea, given that color was first mentioned in the first screen of the instructional program. The accompanying diagram, showing a prism dispersing white light into a spectrum, although meant to be no more than schematic, could be misleading as it is not an accurate representation of the refraction of light passing through a prism.
The concept of intensity is explained by demonstrating the kind of light produced by automobile headlights on high and low beams, but is not related back to the principles of the spectrophotometer at any stage.
The following screen explains that light can interact with matter by reflection or scattering or by being absorbed "to excite the molecule," the latter being the basis of spectrophotometry. It is of interest to note that previously in the program, the excitation of electrons had been the focus of the explanation of absorption. No mention had been made, by this stage, of the fact that some light may be transmitted through the substance.
A schematic diagram showing transmission (a term introduced for the first time at this stage) of some parts of white light through a chlorophyll A solution follows, with the comment, "the interaction of light is with individual chromophore molecules in the solution." Regions of absorbed and transmitted light are clearly indicated in the diagram, which is quite instructive.
Two following screens provide a more detailed explanation of Events at the Molecular Level, and optional branches provide two effective animated diagrams showing how the absorption of a photon may alter vibrational states and electron atomic orbital positions. Presentation in these subsections is very clear and illustrated with effective graphics.
In the next part of this Detailed Look, both the infrared (why?) and visible spectra for chlorophyll are displayed. Although it is not the first time that the word "chromophore" has been used in the program, the first link to the dictionary appears here. The term is defined in the dictionary as "a distinctive grouping in a molecule that can absorb ultraviolet or visible light," although earlier the term has been used to mean the molecules in solution with which the light interacts.
The subsection concludes with a screen containing a significant Food for Thought question, cutely pasted on a simulated yellow self-stick note: "Why is the absorption spectrum continuous and not discrete?" No answer to this question is provided, and no explanation is offered, to what must be a puzzling phenomenon to students approaching spectrophotometry for the first time, after having studied atomic spectra. Finally the structural formulas and absorption spectra of chlorophyll A and B are compared in order to illustrate how a slight change in structure may produce a significant change to the absorption spectrum.
The module Spectrophotometer describes, with well-presented graphics, the components of the instrument and also details several models of this instrument available commercially; an excellent way to link the software to reality. In the final screen, the model spectrophotometer used in the simulation to follow (Practice module) is presented with detailed step-by-step instructions on how to operate this simulated instrument. This sequence would be valuable for any student coming to use either the simulation or a real instrument for the first time.
The quantitative aspects of absorption are treated
in a separate module, Quantitative Spectrophotometry: Beer's Law.
There are three subsections: Background, Development (of
the mathematical relationship), and Statement and Applications.
The effects on absorption of concentration, path length, and wavelength
are explained with appropriately labeled interactive graphics. These illustrate
the concepts effectively. The simulated blackboard and chalk, with which
the mathematical derivation of the linear relationship between absorbance,
concentration, and path length is established, is a novel touch.
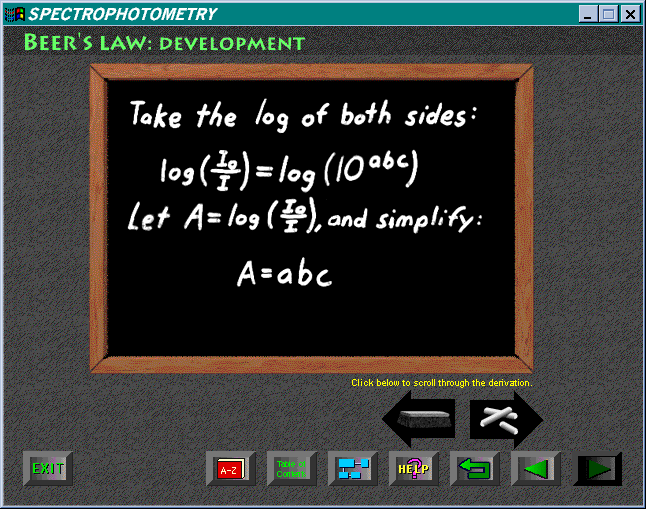
The treatment in this module is comprehensive and thorough. However, there is again a lack of precision in the use of terms. Absorptivity is used to refer to three different constants, and absorption is used several time when the correct term to use is absorbance.
The Practice module, when selected from the initial menu, loads the spectrophotometer simulation from the CD-ROM. If another module is selected while using Practice, one must reload the module.
The realistic presentation of the model spectrophotometer
is most impressive. The initial screen appears in Figure 5.
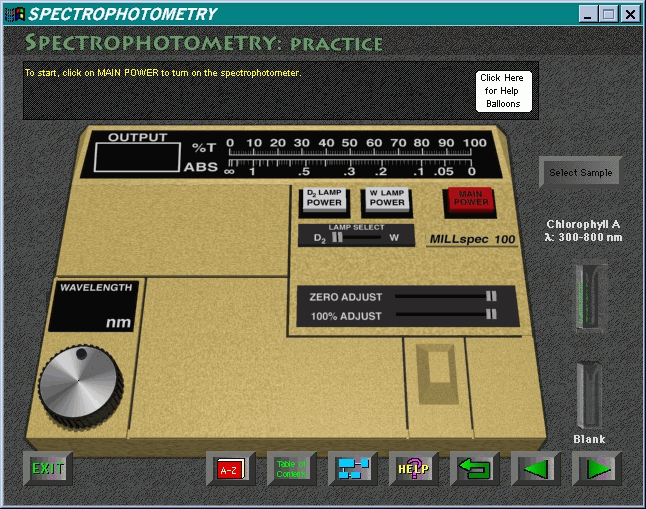
Turning on the machine (by clicking on the main
power switch) removes the lid to expose the internal workings of the simulation
model. This view is most instructive. Figure 6 shows the screen after the
tungsten light source has been switched on (by clicking on the appropriate
power switch) and the zero adjustment made by moving the slider control
with the mouse.
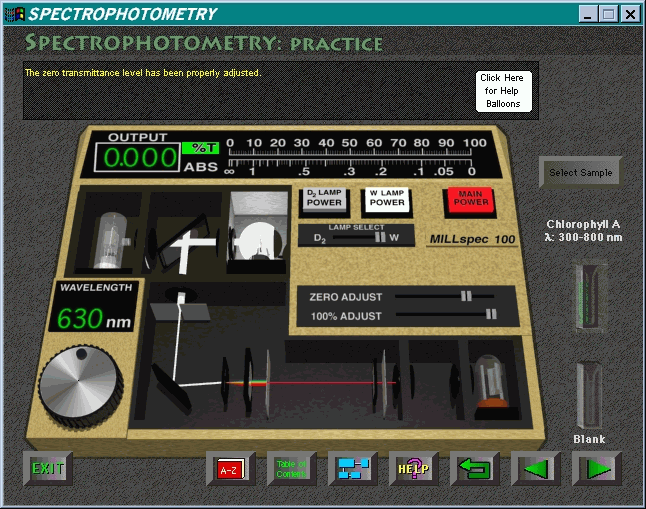
Clicking on the blank cuvette loads it into the instrument; calibration for 100% transmission can then be simulated. Clicking the cuvette labeled "Chlorophyll A" will replace the empty cuvette with this sample, and provide a reading on the meter of either the percent transmission or, on the digital display, of the absorbance. An approximate analog display of transmission and absorbance is presented simultaneously by the line graph to the right of the digital display, a helpful addition to assist comprehension of the relationship between these two concepts. At 630 nm (the default starting wavelength), 83.81% transmission was consistently obtained for the chlorophyll A sample.
At this point, an obvious activity for a user of this software would be to make a series of measurements to produce an absorption spectrum for chlorophyll A. However this will be very tedious as it is necessary to readjust the zero and blank calibration readings each time the wavelength is changed (using the mouse to wind the knob either clockwise or counterclockwise). Even rough readings will be suspect , as the knob on the simulated model and parts of the area around it (below and above-right of the knob) are "hot," and even a click in this area will alter the reading on the digital meter (but not on the analogue display). This is a pity and makes what would be a valuable learning exercise probably too time-consuming to be worthwhile.
Six different samples: chlorophyll A, chlorophyll B, and b-carotene (which have absorption peaks in the visible region), and tyrosine, aldolase and DNA (which have them in the UV region) can be selected for examination. Absorption spectra for each can be viewed. Unfortunately the spectral data used in the simulator for b-carotene are in the region from 200 to 325 nm, and not in the visible region as shown in the spectrum provided when selecting this sample to investigate.
Also 5 samples of solutions of an iron(III) complex at varying concentrations from 0.5 mM to 8.0 mM are provided for investigation. With the wavelength set at 286 nm (determined by trial and error after examining the absorption spectrum provided) the following readings were obtained for three samples, illustrating effectively the relationship between absorbance and concentration—a worthwhile activity: 1.5 mM, absorbance = 0.119; 3.0 mM, absorbance = 0.239; 6.0 mM, absorbance = 0.480.
After each selection of a new sample to be examined, the spectrophotometer module must be reloaded from the CD-ROM, and one waits several seconds before the use of the instrument can be resumed.
The simulation has great potential as a learning aid, but is presented as practice rather than providing a number of investigative activities such as producing an absorption spectrum, or determining the contents of an unknown solution or the concentration of a solution of a known compound. Such additional activities would be very worthwhile.
The program operated without any problems; there
were no crashes. Unfortunately failure to pay greater attention to detail
in the presentation of the instructional material, especially in the use
of language and even spelling, and a lack of consistency among different
aspects of the software (such as the dictionary and the main program modules)
detract from its overall effectiveness. For example, there are typographic
errors in the main body of the program (e.g., "coppertetraammine" and "tramsittance")
and in the dictionary ("coresponding" and a lower-case 'ell' as the symbol
for wavelength). Dictionary definitions are not always consistent with
the main modules (e.g., chromophore is defined in one as a light-absorbing
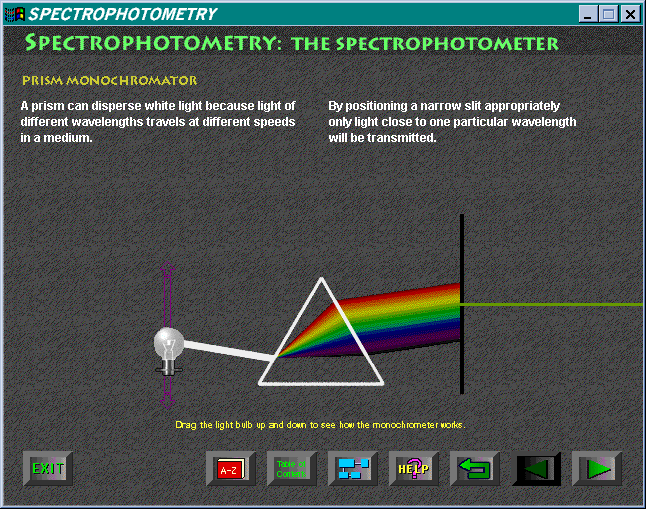
molecule and in the other as a part of such a molecule). The monochromator
of the spectrophotometer is said to "select a small range of wavelengths
of light" in the main program, but is defined as "a device for measuring
the spectrum of incident light" in the dictionary. The diagram explaining
the use of a prism-based monochromator is scientifically incorrect (Figure
7). It is a great pity that the overall value of the software is depreciated
by this lack of attention to detail in some instances.
The simulation of a spectrophotometer is brilliant, but for some practice tasks one might like to undertake, it is a little tedious to use. Providing a series of investigatory activities (simulated experiments) would have increased the range of applications for this software.