 |
Vol. 3 Iss. 4
The Chemical Educator © 1998 Springer-Verlag New York, Inc. |
ISSN 1430-4171
S 1430-4171(98)04223-1
|
 |
Vol. 3 Iss. 4
The Chemical Educator © 1998 Springer-Verlag New York, Inc. |
ISSN 1430-4171
S 1430-4171(98)04223-1
|
Beginning Organic Chemistry 2 - Workbooks in Chemistry, by Graham L. Patrick. 312 pp, softcover. Oxford University Press, Oxford, England. 1997, ISBN 0-19-855936-4.
Beginning Organic Chemistry 2 is the second of two by the same author. The book follows the standard workbook approach of "question—space for the student's answer—correct answer and explanation."
The book is divided into fourteen sections, each dealing with a different topic. The bulk of the text discusses nucleophiles, electrophiles, and their chemistry (seven sections overall, 228 pp). Other topics include reaction mechanisms, acids and bases, acidic protons next to carbonyl groups, elimination reactions, reductions and oxidations, and radical reactions.
The first three sections introduce the basic material required for understanding the remainder of the text. The definitions of a nucleophile, electrophile, nucleophilic center, and electrophilic center are covered in the first section. The second section deals with reaction mechanisms and the use of curved arrows to represent these mechanisms. The third section is a brief one, discussing the classification of reactions. These concepts are well discussed, and the student should master these sections before continuing, as these concepts are heavily used throughout the text.
The section on reaction mechanisms is very well covered, and includes discussions about some of the errors students often make when starting to draw reaction mechanisms (e.g., an arrow coming from a proton instead of to a proton). One question asks the students to examine three mechanisms for the mistakes made therein. This examination of the work should make the students more aware of what to look for in writing a mechanism, and also make the students aware of how questions might be marked!
The author takes a very rigid approach in showing how the mechanisms are to be drawn, specifically, when drawing an arrow forming a new bond between two species. "If the electrons are being used to make a new bond, the mechanism arrow must point to where the center of the new bond will be." This is formally correct, but will it lead to student understanding? Two problems are foreseen.
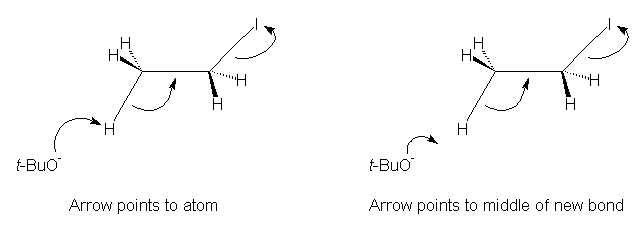
First, will a beginning student recognize that the bond is being formed between the two atoms? In some of the mechanisms drawn, there is a large amount of space between the two bonding atoms, and the student may not easily visually connect the two atoms as part of the whole mechanism. In other (rare) cases, the arrow ends closer to a hydrogen atom, when it is a carbon atom that is being attacked. This can be confusing even to an experienced chemist. Second, most modern presentations take the less formal approach to the use of curved arrows. In a short survey of 16 textbooks (beginning through advanced organic chemistry) and several journal articles, the mechanisms were drawn with the arrow pointing directly at the atom to which the bond was formed in all but one of the textbooks. It is felt that this is the preferred method of using curved arrows (despite the formally incorrect nature), because the student can see quickly and easily which atom is being bonded. This is especially true when larger complex structures are used. Perhaps it is time that authors and/or editors agree on how mechanisms are to be presented.
The section on acids and bases is probably the weakest in the book. The beginning of the section focuses on introductory acid–base theory rather than on the chemistry of organic acids and bases. HF is presented as a strong acid, though its Ka (3.5 x 10-4) is only 20 times stronger than ethanoic acid (Ka = 1.75 x 10-5). The most useful part of the section, resonance effects on acid strength, does cover the concept, but at a very slow pace.
The next three sections of the book cover electrophilic additions and substitutions. The coverage here is excellent. The material on electrophilic additions begins simply, and increases in complexity at an appropriate pace. The sections on electrophilic substitutions are likewise arranged, and culminate in questions that have the student think about synthetic planning. These questions were well thought out, and are some of the most difficult in the book.
Nucleophilic additions and substitutions also cover three sections, and the coverage here is also excellent. These sections contain the best question-and-answer pair in the book. This is a brief look at "examination technique," using questions about nucleophilic addition. Five questions are asked of the student, and each is worth so many "marks." The answer to the questions are of course provided, but so is a written commentary as to the assignment of the marks! This allows the student to gain an understanding of how to present an answer in an exam situation. An excellent teaching tool; there should be more of these questions in the book.
The final four sections of the text cover acidic protons next to carbonyl groups, elimination reactions, reductions and oxidations, and radical reactions. While the questions are adequate, these sections are too brief to provide in-depth learning, and are better used for review.
Visually, the text is well laid out. The equations are large and clear, and there is enough space for the student to write their answers in the book. There are few typographical errors in the text. The mixture of serif and sans-serif characters in the equations may give typographers cause for concern, but should not bother the average student.
Despite some weak sections, this text is strongly recommended for any teacher who wishes their students to master the concepts of nucleophilicity and electrophilicity in organic chemistry.