 The
Chemical Educator, Vol.7, No. 5,
Media Reviews, © 2002 Springer-Verlag New York, Inc.
The
Chemical Educator, Vol.7, No. 5,
Media Reviews, © 2002 Springer-Verlag New York, Inc.
Media Reviews
 The
Chemical Educator, Vol.7, No. 5,
Media Reviews, © 2002 Springer-Verlag New York, Inc.
The
Chemical Educator, Vol.7, No. 5,
Media Reviews, © 2002 Springer-Verlag New York, Inc.
Media Reviews
Sugar, Quartz, Diamonds: Crystalline Perfection (The Science Bag). Dennis Bennett. 56:42-minute VHS videocassette, Catalog No. CRY123, 1995. $89.00 plus shipping & handling (7% of catalog price with a minimum of $6.00). Order from: Blue Sky Associates, 1208 Bridge Street, Grafton, WI 53024;Telephone: (262) 377-1398; FAX: (262) 377-7750; email: science@blueskyassociates.com; Web site, which contains an order form and brief descriptions of current titles: http://www.blueskyassociates.com.
The Science Bag is a popular series of Friday night television programs presented by chemists, biologists, geologists, anthropologists, mathematicians, meteorologists, and physicists that has been viewed by more than 125,000 persons. It was begun by faculty members at the University of Wisconsin-Milwaukee in 1973 to make science more accessible to the general public. Blue Sky Associates, with the motto “Your Partners in Science Teaching,” was founded in 1989 to market videotapes of selected programs of “The Science Bag.”
The approximately 45-to-55-minute tapes are divided into two segments by a designated breakpoint for greater flexibility in presentation. Each program is distinctly different from the others in focus and format, but each makes extensive use of a wide range of visual material and demonstrations, many of which are quite elaborate and beyond the scope of the usual classroom presentation, while others can be performed as follow-up activities. The tone and style are down-to-earth, often fairly casual and laced with humor, with audience participation and involvement. The videos are intended for an audience of non-scientists of varying age ranges and are all suitable for middle and high school classes as well as for introductory or survey college courses.
The video under review here deals with the following topics:
I. Composition of crystals
II. Formation of crystals
III. Structure of crystals
IV. Symmetry in crystals
V. Properties of crystals
VI. Quartz and ice crystals
VII. Importance of the structure of ice
VIII. How to grow crystals
From the moment that Dennis W. Bennett, a crystallographer and professor of chemistry at the University of Wisconsin-Milwaukee, appears in his blue T-shirt adorned with images of huge, white, snowflake crystals, his delight in his subject is both obvious and infectious. The primary goal of his presentation is to convince his audience that the structure and properties of crystals—the way that they look and behave on the outside—are related to the unit cell—how they look on the inside. With charts and pictures of the atoms, molecules, and ions that comprise crystals he vividly illustrates how these elements are attracted to each other and pack together as closely as possible, not randomly but in ordered, repeating arrangements.
By pressing a metal clicker that sends a shock wave through a supersaturated solution of sodium acetate contained in a plastic bag, Bennett shows how to grow crystals quickly and in this case how the crystallization process is exothermic. He strikingly explains the symmetry operations by asking four young volunteers from the audience to try to “tile a bathroom floor” using large cardboard pentagons, hexagons, octagons, and triangles so that no holes are present. Only a few symmetry operations (2-, 3-, 4-, and 6-fold) can accomplish this. He extrapolates the procedure from two to three dimensions, and with three-dimensional models he lucidly explains the different unit cells (cubic, tetragonal, orthorhombic, monoclinic, triclinic, and hexagonal) and their interrelationships. He explains that crystals cleave or break corresponding to their internal symmetry, and he shows how diamonds are cut and how their properties differ from another form of carbon—graphite. In another demonstration of different forms of the same substance he contrasts quartz and sand (both SiO2), employing an ingenious, homemade contact goniometer made of two hacksaw blades and a bolt. He discusses why glass shatters irregularly at odd angles and the shards are sharp (the amorphous solid possesses no regular internal structure and therefore has no cleavage planes).
A spectacular demonstration that we hadn’t seen previously was what Bennett called the “methane mambo,” involving soap bubbles and natural gas, forming an airy and ever-elevating column, which is then ignited, resulting in a “poof.” Because the formation of calcium carbonate stalactites and stalagmites in limestone caves is too slow to be shown, Bennett demonstrates the formation of a “stalagmite” as a supersaturated solution of sodium acetate crystallizes and absorbs water incorporated into the crystal structure as water of crystallization (NaC2H3O2·12H2O). Among other crystals that he exhibits and discusses are copper sulfate (CuSO4·5H2O), sodium chloride (NaCl, table salt), alum (K2SO4·Al2(SO4)3·24H2O) with its beautiful octahedra, Rochelle salt (NaKC4H4O6·4H2O), potassium ferrocyanide (K4(FeCN)6), and nickel sulfate (NiSO4·7H2O).
By immersing ethylene glycol in liquid nitrogen, Bennett demonstrates that most liquids shrink and become denser on freezing, and he contrasts this behavior with that of water, which expands on freezing (ice floats in water), which he ascribes to hydrogen bonding. He discusses the crucial importance of this unique property of water, which makes life as we know it possible on earth. He demonstrates the formation of rock candy and gives specific directions for this process, using a string suspended in a cup of sugar (sucrose) and water. He fails to wear the politically correct goggles, perhaps because he already wears glasses. He concludes his presentation by showing how even unsymmetrical objects can pack together symmetrically.
We are pleased to recommend as a valuable resource for science teachers this informative and engaging video. It makes complex science not only understandable, relevant, and interesting, but also lots of fun.
George B. Kauffman and Anastacia Melendy,
California State University, Fresno,
georgek@csufresno.edu and krazzychic19@aol.com
S1430-4171(02)05602-3, 10.1007/s00897020602a
Scientific American’s “The Amateur Scientist”: The Complete 20th Century Collection on CD-ROM. Shawn Carlson and Sheldon Greaves, editors. CD-ROM, 2000. ISBN 0-9703476-X. $39.99. Order from: Tinker’s Guild, 410 Tiogue Avenue, Coventry, RI 02816; Telephone: (401) 823-7367 (Voice and FAX); Toll-free, (888) 875-4255; email: info@tinkersguild.com; for purchase orders from schools, museums, and other educational institutions: FAX: (877) 503-0148 or mail to above address; Web site, which contains an order form and complete list of all articles, arranged by subject: http://www.tinkersguild.com/.

Many chemical educators chose their career largely because of their home experiments carried out at an early age. Thus this CD-ROM will be of exceptional interest to those of us in this category.
Because “The Amateur Scientist” column, the premiere publication for hands-on science, began appearing in Scientific American magazine in 1928, it has inspired innumerable amateur experiments, stimulated hundreds of thousands of science fair projects, launched careers in science and technology, and enjoyed a place of honor in classrooms and school libraries all over the world. Although always affordable for an amateur, the column’s projects are frequently sophisticated and elegant. Some have been so innovative that they have established new standards in a field. Numerous professionals borrow material from the column to find low-cost solutions to real-world research problems.
Shawn Carlson, a 1999 recipient of a MacArthur “genius” award for his contributions to amateur science, wrote “The Amateur Scientist” until March 2001. In 1994 he founded the Society for Amateur Scientists, a nonprofit organization to help amateur scientists of all ages and experience levels become involved in research projects, often working with professional researchers (5600 Post Road, #114-341, East Greenwich, RI 02818; Tel.: (401) 823-7800; Web site: http://www.sas.org). Sheldon Greaves, a contributing editor of Amateur Scientist Bulletin, the official newsletter of the Society for Amateur Scientists, was a technical writer for several Silicon Valley software companies and a research associate at the University of California, Berkeley. The two have collected and edited a comprehensive anthology of all “The Amateur Scientist” columns from its inception through November 1999 on a single CD-ROM, justifiably advertised as “the ultimate resource for hands-on science and most complete compendium of science projects ever assembled.”
System Requirements: PPC or Pentium processor; 32 MB RAM minimum; Java-aware browser such as Netscape 3.1 or higher (4.0 or higher recommended) or Internet Explorer 3.0 or higher (This browser-based document will also operate on Unix and Linux platforms equipped with a Java-aware Web browser).
The collection contains every one of the 802 columns (with text and pictures for each column) that have appeared in “The Amateur Scientist” (by Albert Ingalls, C. L. Strong, Jearl Walker, Shawn Carlson, and guest authors, updated, amended, and corrected by Shawn Carlson and invited writers); “The Amateur Astronomer” and “The Amateur Telescope Maker” (both by Albert Ingalls); “Procedures in Experimental Physics” by John Strong, a classic text and much sought-after reference on instrument making; special commentary by Shawn Carlson to provide key insights on some of the more popular projects; additional detailed technical information to supplement individual topics; a brief history of the columns and the persons who wrote them; extensive materials on building scientific apparatus such as vacuum techniques and glass-blowing; and a detailed glossary. It includes directions on such topics as how to separate DNA in the kitchen sink or build a cyclotron, X-ray machine, or a simple seismograph to record earthquake waves at home. It shows home-based experimenters how to make original discoveries using only inexpensive materials.
In all, the CD-ROM includes more than 1000 projects in almost every field of science; voluminous supplementary materials that are difficult to find as well as more than 1000 pages of helpful science secrets that have never appeared in Scientific American; resource links to the location of necessary materials; and a large number of shareware, freeware, and demoware software titles. It allows the user to search for words or phrases anywhere in the text and to select projects by subject, age suitability, cost, hazard level, relationship to other projects, and other criteria.
It is the indexing and the organization of this software that makes it so useful and so different from other sources of scientific projects. The user of the program can search for an experiment by subject, content, or date (decade). Subject links are organized according to the following subjects: Archæology, Astronomy, Biology, Chemistry, Earth Science, Mathematics, Physics, Technical Notes (supplementary material that never appeared in Scientific American), and Tools and Techniques. Each link takes the user to a list of the columns with projects on that subject. Because some columns contain more than one project, they appear under more than one subject heading. Searching by decade is probably the easiest way to search the library of information and find experiments.
Many Chemistry columns deal with chemical analysis such as: “Exploring Chemical Bonds,” “An Inexpensive Homemade Polarimeter Can Analyze Optically Active Compounds,” “How Can the Amateur Detect Metals in Air, Liquids or Solids?,” “Making a Refractometer for the Identification of Liquids,” “Making an Apparatus That Will Measure the Acidity or Alkalinity of Solutions,” “How to Construct a Gas Chromatograph That Can Measure One Part in a Million,” “On Constructing and Using a Photoelectric Colorimeter for Various Chemical Analyses,” and “How to Build a Polarograph, a Sensitive Instrument for Making Chemical Analyses.”
Others deal with chemical properties such as: “Chemical Systems That Oscillate Between One Color and Another,” “How an Amateur Can Construct a Model of an Enzyme at Modest Cost,” “Molecular Models and an Interferometer That Can Be Constructed at Modest Cost,” “How to Blow Soap Bubbles That Last for Months or Even Years,” and “On Equipment to Study Freezing and on Growing Crystals of Salt.” Among laboratory techniques one can find columns such as: “The Kitchen as a Lab,” “Painting in Color Without Pigments,” “Retracing the Steps By Which Aluminum Metal Was Initially Purified Back in 1886,” “The Physics and Chemistry of Lemon Meringue Pie,” “Mead, the Drink of the Vikings, Can Be Made (Legally) By Fermenting Honey in the Home,” and “How to Make and Electrochemical Cell and Also an Unusual Kind of Sundial.” Most of the Scientific American columns deal with constructing apparatus with which various experiments can be performed.
Each experiment is accompanied by the following ratings:
Cost: an estimate based on current prices, and it may differ from the costs given in the columns: $0–100 less than $100; $100+ more than $100.
Difficulty: an estimate of the time, experience, and skill needed to build a project successfully: 1: easy, no special skills needed; 2: moderate, a young person may require parental guidance; 3: intermediate, some experience required; 4: difficult, for advanced amateurs only; and 5: very difficult, special skills needed. Most of the experiments have a difficulty rating of either 2 or 3, and many of these appear to be very challenging. Experiments with a rating of 5 include “A High School Student Builds a Plasma Jet Generator That Attains Solar Temperatures” and “How a Persevering Amateur Can Build a Gas Laser in the Home.”
Danger: to alert the experimenter to chemical, electrical, biological, or physical hazards; 1: No hazards; 2: minor injury possible; 3: serious injury possible; 4: possibly lethal (adults only).
Utility: an assessment shown by logos of a project’s usefulness for science fairs and home-based research: Cool project: a good contender for science fairs and home experiments, uses obsolete technology but could be updated, explores a problem that is now well understood but might still be fun or educational to do, and historical interest.
This CD-ROM is the ultimate resource for amateur scientists, hobbyists, science fair participants, educators, home schoolers, professionals seeking novel solutions to research problems, teachers, students, and anyone interested in hands-on science experiments and projects.
George B. Kauffman and Brian Fischer,
California State University, Fresno,
georgek@csufresno.edu and bef03@aol.com
S1430-4171(02)05603-2, 10.1007/s00897020603a
The Standard Deviants Present: Chemistry, Part 1. The Standard Deviants Academic Team including David Rowley and David Ramaker. 1-hour-56-minute VHS videocassette, 1998. $19.95. Order from: Cerebellum Corporation, 2890 Emma Lee Street, Falls Church, VA 22042; Telephone: (800) 238-9669; email: info@cerebellum.com; Web site: http://www.cerebellum.com.

Since its founding in 1993 the Cerebellum Corporation, one of the United States’ leading producer of educational videos and DVDs, has combined solid educational content with cutting-edge technology and top-notch writing in its attempt to make the most difficult high school, college, and university subjects comprehensible to students. Each video is written by an academic team of professors from leading American universities—in the case of chemistry, David Rowley and David Ramaker of George Washington University.
As the video begins the viewer is welcomed to “the supercharged world of chemistry” and is assured that he or she will “rock and roll with all the chemical concepts that you will need to know.” The viewer is cautioned that the video is not a substitute for a formal course and is urged, “Attend your classes! Listen to your professors! “Eat lots of gouda cheese! Go to class!” The question “What is chemistry?” is asked and answered, and the viewer is cautioned that nomenclature makes the subject seem more formidable than it really is (“Learn the language!”) and that chemistry is really easy to learn—an assurance that is repeated throughout the video. Detailed instructions on how to use the video are also provided.
In a multiplicity of interesting venues, most of which avoid the formal classroom or lecture hall in favor of bedrooms, police stations, the campus of Atomic Mass University (AMU), and other unlikely locales, the program deals cleverly with the following topics: States of Matter: Liquids, Solids, Gases; Physical Properties of Matter; Chemical Properties of Matter; Heterogeneous Matter; Homogeneous Matter; Atoms; Compounds; Molecules; The Periodic Table; The Metric System; SI Units; Significant Figures (nicknamed “sig figs”); Scientific Notation; Unit Factor Method; Stoichiometry; Balancing Chemical Equations; Law of Conservation of Mass; Atomic and Molecular Weights; Mass Number; Atomic Number; Isotopes; Ions; Atomic Mass Units; Moles; Molecular Mass; Molar Mass; Percent Composition; Empirical Formulas; Solution Stoichiometry; Molarity; Titrations; Limiting Reagents; and Yields. Actually, the coverage is much more extensive, for the above list includes only the main topics.
To say that the approach is an unconventional one would be an understatement. The wild and wacky program abounds with zany comic-strip-like characters with Brooklynese or other accents. It features a flying superhero in elaborate costume and cape (Helium Man), Wild Worm, and numerous other unusual figures. Short scene follows short scene with reckless abandon. The frequent scene changes involve a dazzling variety of narrators who often speak in mock-heroic style, at a frantic and frenetic pace, much in the manner of television commercials or 30-second sound bites. The action is so fast and furious that the viewer will frequently need to use the pause and rewind buttons to follow all the chemical topics.
Typical TV-like settings include a court scene presided over by Judge Hangem with an attorney prosecuting a miscreant for violating the law of conservation of mass and the “Cooking with Professor Rowley” show, where Rowley synthesizes magnesium oxide from magnesium. The approach is often “hip,” “corny,” or “cutesy,” but always vivid, striking, and attention-getting. A statement that the neutron is neutral—like Switzerland—features a Swiss miss with blonde braids in peasant costume. The mole makes it easier to deal with “humongous” numbers: “a mole of beer nuts would cover the surface of the earth.” In a discussion of stoichiometry reactants are called “starting stuff.” On one occasion the narrator calls time out for “some complete utter foolishness.” When detailed calculations are explained, the frenetic pace and scene changes are moderated, and the treatment becomes more conventional. The video ends with a summary of the material that has been discussed and the assurance that, “You’re ready to rock and roll with chemistry.”
With its fun approach to serious education, clever skits, mnemonic devices, and high-tech computer graphics, the video might be described in TV terms as “Laugh-In” or “Sesame Street” meets Alan Alda’s “Scientific American Frontiers” or Roald Hoffmann’s “The World of Chemistry.” In today’s vernacular it could be proclaimed as “something else,” “far out”, or the ultimate accolade—“cool.”
The video is well organized and makes a good study resource for almost any introductory chemistry course. The topics are explained clearly, and the tape features a number of creative parts. However, once the scientific information stops, and the banter between the different characters begins the tape may try the more serious viewer’s patience.
In a discussion of how to calculate a weighted mass average for isotopes, the narrator incorrectly states that an atom of hydrogen contains a proton and neutron, while an atom of deuterium contains a proton and two neutrons. However, the graphs for the mass of hydrogen and deuterium are both correct so the average viewer probably wouldn’t notice the mistake. Also, in a section dealing with the periodic table the narrator states correctly that on descending a group atoms become larger but incorrectly that on proceeding left along a period atoms become smaller. In one equation sodium chloride is written “NaCL,” and elsewhere bases are referred to as “alkalines.” Furthermore, safety goggles are not used.
A pessimist might consider the video a pandering to and a sad commentary on a generation of students raised on TV with an expectation that everything should be easy and accomplished with minimum effort. An optimist might consider it as a reflection of present-day society and an ingenious way of making high-tech state-of-the-art animation and techniques and the hype and hoopla of TV to reach students comfortable with TV formats. Societies of educators and scientists seem to favor the optimistic view. Standard Deviants titles have won 19 Telly Awards for “Best Non-Broadcast Educational Videos” (The Chemistry video is a 15-time Telly winner) and have been named to the “Best Films and Videos” list by the American Association for the Advancement of Science (AAAS).
All things considered the video does a good job of explaining in adequate detail the fundamental concepts of chemistry in an easily understood manner, and it makes a useful study aid for most introductory students. However, it is not something suitable for a large group to watch together because the dialogue and shenanigans between the characters is so distracting that in a large group this extraneous material is all that would be discussed, and consequently the subject matter would be neglected. When the video is used repeatedly as a personal study aid, the dialogue, which may have served a legitimate purpose during the initial viewing, can be eliminated by fast forwarding to the topics of concern. A guide showing the exact times on the video for the various topics would be useful for this purpose.
The video can also be used as an introduction to or as a review of a particular topic in introductory chemistry. We recommend it to students, teachers, parents, school boards, home schoolers, libraries, continuing education students, and knowledge seekers in general. Enjoy!
George B. Kauffman and Brian Fischer,
California State University, Fresno,
georgek@csufresno.edu and bef03@hotmail.com
S1430-4171(02)05604-1, 10.1007/s00897020604a
The Chemistry Animation Project. A series of VHS videocassettes for nonprofit educational use only. Nathan S. Lewis, project director. VHS videocassette, 1994-. $19.95 plus postage and handling. Order from: California Institute of Technology Bookstore, Mail Code 1-51, Pasadena, CA 91125; Telephone: (800) 514-BOOK; FAX: (626) 795-3156; email: citbook@caltech.edu.
Because chemistry concerns the spatial relationships between invisible, continually moving objects, badly drawn diagrams can befuddle rather than enlighten students, making simple topics more complex and complex topics incomprehensible. This can make chemistry appear more difficult than it actually is to students, many of whom consequently abandon their study of “the central science.”
In 1991 Caltech chemistry professor Nathan S. Lewis, who admits that he “can hardly draw anything,” realized that no one had ever put atomic orbitals, a basic concept in introductory chemistry courses, on a computer in good visual form. He recruited Andre Yew and J. Alan Low, two SURF (Summer Undergraduate Research Fellowship) students, who, by the summer’s end, had created a 10-minute-42-second videocassette, “Atomic Orbitals,” thus initiating Caltech’s Chemistry Animation Project (CAP). With major funding from the Howard Hughes Medical Institute, the National Science Foundation, E. I. DuPont de Nemours, Inc., the Camille and Henry Dreyfus Foundation, the Annenberg Foundation, Biosym, Inc., the John Stauffer Charitable Trust, Alias/Wavefront, and the California Institute of Technology, the CAP is using state-of-the-art computer animation to produce videocassettes showing scientifically accurate, three-dimensional depictions of fundamental chemical concepts to help high school, college, and university students to visualize what cannot be seen.
With the motto “Visualizing the Chemical World,” each videocassette in the series offers insight into chemical phenomena for instructor and student alike with intuitive three-dimensional accuracy. Professional narration and music as well as animation by Hollywood film animators Nick Oldark, Scott Fink, and Judy Kriger are provided for each video.
The videos use vivid analogies and humor to capture the interest of even the most unmotivated students, such as flying calipers that measure atomic radii and periodic tables whose squares turn colors and become bars whose heights are proportional to whatever property is being illustrated. In a segment dealing with ionization energies an organ grinder’s monkey cranks an organ, which is actually a force meter, to tighten a rope attached to an electron. The 10-to-15-minute user-friendly modules are designed for teachers to use in whatever order they desire. Lewis hopes that instructors will edit the videos on their own VCRs to produce tapes that suit their own needs. He also believes that computer animation is adaptable as a teaching tool for sciences other than chemistry.
Preparing the videos is so labor-intensive that only about a half-hour of broadcast-quality animation is produced annually. The following videos are available: “Atomic Orbitals” (10 min 42 s.) by Yew and Low; “VSEPR” (11 min 18 s.) by Mark Huber and Corinna Garcia; “Crystals” (12 min.) by Todd Allendorf; “Stereochemistry” (13 min.) by Michael Medaglia, Huy Lee, and David Zito; “Molecular Orbitals” (14 min.) by Anthony Molinaro and Andre Yew; “Diels-Alder Reactions” (9 min.) by Tim Uy and Anil Roopnarine; “Nucleophilic Substitution” (9 min.) by Chris Bryant and Sean Upchurch; and “Periodic Trends.” Videos currently in production are: “Binary Crystals,” “Hybridization/Resonance,” and “Spectroscopy/Molecular Motions.”
The first two modules, “Atomic Orbitals” and “VSEPR,” are typical of the series. Understanding these two topics can be a very difficult task for high school, college, and university students. The first module, with the second movement of Antonin Dvořak’s “New World Symphony” (“Going Home”) as background music, vividly illustrates the s, p, d, and f orbitals and their role in the organization of the periodic table and the properties of chemical bonding. The size, shape, and orientation of each orbital is shown, superimposed over the xyz axis and its position relative to some of the lower orbitals. The electron probabilities are also shown in shaded gradients, and nodal planes are explained as regions where no electrons will be found. For a student who might have just been exposed to the idea that the electron does not orbit the nucleus in a discrete planetary-like orbit these animations clearly provide an explanation as to where the electron would probably be found.
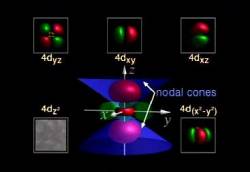
The periodic table is also referred to, with each orbital block given a color and each element shown as a shade of that color. Furthermore, the valence orbital is indicated for each period. For example, the elements from gallium (at. no. 31) to krypton (at. no. 36) all have valence electrons in the 4p orbital, and this is shown for this period on the periodic table. This treatment is performed for the entire table.
VSEPR (valence shell electron repulsion), the theory first described by Nevil V. Sidgwick and H. M. Powell and then developed by Ronald J. Gillespie and Ronald S. Nyholm, provides a relatively simple and effective method for predicting the shapes of molecules. The first molecules dealt with in the video are those with two atoms bonded to a central atom and with no lone electron pairs. This situation, of course, yields a bond angle of 180° and a linear shape.
The video demonstrates and uses the principle of steric number (the sum of bonding electrons and lone pair electrons) to help predict the shape of a particular molecule. The narration then explains that the electron pairs and the atoms want to be as far apart from each other as possible, and this is shown with the molecule superimposed on a sphere to help demonstrate the repulsion with subsequent bond angles that result in space. Water is one of the examples used, and it is shown in a bent configuration because of the two electron pairs. Molecules such as BH3, NH3, and ClF3, and those with double bonds are discussed.
Also explained are the equatorial and axial bonds and positions and their relevance in determining the shape of the molecule. Lewis dot structures are also briefly shown just before the bonds are drawn between the atoms in a given molecule, a treatment that helps to reinforce the fact that a single bond consists of two shared electrons.
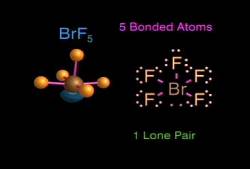
By the end of the video every case from a simple linear molecule, with two atoms bonding to a central atom, to the complex structure of an octahedral molecule have been discussed with a final image showing all the shapes on the screen together. The animation and narration are exemplary in their explanation of these concepts. The visual aspect of the animation makes something that is not otherwise easily understood much easier to understand.
An online forum for ordering information, product information and availability, new title announcements, and feedback can be accessed on CAP’s Web site: http://bond.caltech.edu or obtained by email: cap@bond.caltech.edu. If instructors buy their own personal copies, they may make an unlimited number of copies free of charge for use by their students. Additional information can be obtained from Dr. Nathan S. Lewis, Division of Chemistry and Chemical Engineering, Mail Code 127-72, California Institute of Technology, Pasadena, CA 91125; Telephone: (626) 395-6335; FAX: (626) 795- 7487; e-mail: nslewis@its.caltech.edu.
George B. Kauffman and Hiram William Blanken,
California State University, Fresno,
georgek@csufresno.edu and bmblanken02@attbi.com
S1430-4171(02)05605-0, 10.1007/s00897020605a
Linus Pauling: Scientist and Peacemaker. Edited by Clifford Mead and Thomas Hager. Oregon State University Press: Corvallis, Oregon (http://osu.orst.edu/dept/press), 2001. x + 272 pp. hardcover. 18.3 ´ 26.0 cm. $35.00. ISBN 0-87071-489-9. To order by email: special.collections@orst.edu

Linus Pauling, the internationally acclaimed scientist, educator, humanitarian, and political activist, was characterized by New Scientist magazine as one of "the twenty greatest scientists of all time, on a par with Newton, Darwin, and Einstein." He has been called one of the two greatest scientists of the 20th century (the other being Einstein) and the greatest chemist since Antoine-Laurent Lavoisier, the 18th-century founder of modern chemistry. His magnum opus, The Nature of the Chemical Bond (1939) is one of the most influential and frequently cited scientific books of our century. His advocacy of megadoses of vitamin C for the common cold, cancer, and AIDS is the controversial work for which he is best known to the general public.
His multifaceted achievements include the multidisciplinary use of one science (physics) to explain another (chemistry); his combination of quantum mechanics and X-ray diffraction to win the 1954 Nobel Prize in Chemistry "for his research on the chemical bond and its application to the elucidation of the structure of complex substances;" his seminal contributions to chemistry, quantum mechanics, X-ray crystallography, anesthesia, electronegativity, the structure of proteins and DNA, nutrition, mineralogy, nuclear physics, and immunology; his research on molecular biology and orthomolecular medicine; his work for the U.S. government during World War II; his campaign against nuclear weapons testing, which earned him the 1962 Nobel Peace Prize; and his evolution, encouraged by his wife Ava Helen, from an ivory tower scientist to an ardent and articulate public spokesman on technological issues and the social responsibility of scientists. His life, both scientific and personal, was characterized by controversy, and almost everything about him was larger than life.

Figure 1. Linus and Ava Helen Pauling on the beach at Corona Del Mar, California, 1924 (p 7). From the Ava Helen and Linus Pauling Papers, Special Collections, Oregon State University.

Figure 2. Linus Pauling’s handwritten description of his first meeting with Ava Helen Miller, who was a student in his home economics chemistry class (p 7). From the Ava Helen and Linus Pauling Papers, Special Collections, Oregon State University.
Pauling’s life spanned almost the entire twentieth century (He was born in Portland, Oregon on February 28, 1901). In 2001 a variety of national and international activities, exhibits, symposia, lectures, events, books, medals, models, and other items and events commemorated the centenary of his birth [1]. February 2001 was proclaimed as Linus Pauling Month by Oregon Governor John A. Kitzhaber, who is a physician (MD, Oregon Health Sciences University, 1973). According to Kitzhaber,
· Linus Carl Pauling remains the only person to have been awarded two unshared Nobel Prizes, one for Chemistry in 1954 and one for Peace in 1962.
· Pauling’s efforts to bring about world peace and the banning of nuclear weapons testing, carried out in spite of the disapproval of his own government, established a new base for world peace negotiations.
· Pauling’s scientific genius established the basis of modern chemistry and helped found the discipline of molecular biology, therein setting the stage for major discoveries that have benefited humankind.
· Pauling honored his alma mater Oregon State University, and the people of Oregon with the gift of his papers, books, medals, research models, and memorabilia, a total of more than 500,000 items covering the years 1916–1994.
Governor Kitzhaber also proclaimed February 28, in perpetuity, as “Linus Pauling Day” in the state of Oregon. Each year on that day OSU’s Pauling Heritage Committee coordinates a series of events to focus attention on the remarkable life and career of the university’s most famous graduate.
The idea for a centenary volume to commemorate Pauling’s hundredth birthday originated with Clifford Mead, Associate Professor and Head of Special Collections at Oregon State University’s Valley Library, who oversees the Linus Pauling Collection, which contains Pauling’s complete personal papers and includes hundreds of thousands of letters, articles, photographs, memoranda, and molecular models comprising his entire life’s work. He is also the coeditor of two books on Pauling and the Pauling Papers [2]. Coeditor Thomas Hager is a science writer, biographer, editor, Assistant Professor at the University of Oregon, Acting Director of the University of Oregon Press, and author of two biographies of Pauling [3, 4]. Many of the pieces that appear in the book, a number of which have never been published, were obtained from the collection. Unfortunately, the sources of some of the pieces are not clearly indicated.
Mead decided that because a number of narrative biographies of Pauling were already in print [5, 9], the best approach for a useful and readable commemorative volume would be that of a “mosiac” similar to the centenary volumes devoted to Albert Einstein [10] and Niels Bohr [11], which were collections of first-person accounts, historical reminiscences, illustrations, and brief anecdotes that illuminated their subjects from various viewpoints. Such an approach allowed the editors to use the Ava Helen and Linus Pauling Papers at OSU and make public some of the written documents and photographs that might otherwise be limited to a small number of scholars. Also, Hager includes striking and humorous quotations from interviews that he had held with Pauling’s colleagues, contemporaries, students, and enemies as marginalia in the book. The editors selected the primary and secondary materials according to quality and comprehensiveness in an attempt to produce “an almost cubistic view from many angles—personal and critical, contemporary and historical, first-person and third-person—of one of the central scientists in twentieth-century history.” In our opinion Pauling’s fellow Oregonians have succeeded in achieving their goal.
The collection contains 27 pieces arranged in three main sections plus a short fourth section.
Part I. “Linus Pauling, the Man”
· In “The Roots of Genius” (6 pp), which introduces the volume, Tom Hager succinctly summarizes Pauling’s life and career with an emphasis on the central influences that shaped his sometimes contradictory personality.
· In “A Pauling Chronology” (13 pp) Pauling biographer Robert Paradowski chronicles the main events in Pauling’s life. At the time of Pauling’s birth the discipline of chemistry was dominated by Germans, whereas at the time of to his death on August 19, 1994 at his Deer Flat Ranch in Big Sur, California it was dominated by Americans, a profound change brought about largely by his own efforts.
· In “My Best Friend” (4 pp) Pauling pays tribute to Lloyd Alexander Jeffress, whose friendship was crucial twice in his life—first, when he introduced the adolescent Pauling to chemical experimentation and second, when he and his family encouraged Pauling to continue to attend college despite Pauling’s widowed mother’s opposition. Pauling’s second son is named Peter Jeffress Pauling.
· In “Diary Excerpts” (6 pp) in his 17th year, on August 29, 1917, Pauling proclaims, “Today I am beginning to write the history of my life.” In the last of the 11 entries (October 29, 1917) he wrote of 17-year-old Irene Sparks, “She is the girl for me.”
· In “Interview with Dr. Linus Pauling” (25 pp, the longest piece in the book) Pauling gives detailed answers to a series of wide-ranging questions put to him on November 11, 1990 by Wayne Reynolds, Executive Director of the American Academy of Achievement.
· In a previously unpublished piece, “Summer Employment” (3 pp), Pauling describes the various odd jobs (1916–1923) without which he could not have afforded to attend college. They not only demonstrate his versatility and entrepreneurial ability, but they also taught him some chemistry.
· During his junior year at Oregon Agricultural College (now Oregon State University) Pauling competed in a school-wide oratorical contest with a speech titled “Children of the Dawn” (4 pp). It shows that his optimism and belief in progress through the scientific method were part of his character from an early age (Pauling lost to the senior speaker and tied with the sophomore speaker).
· In “Linus Pauling, the Teacher” (8 pp) eminent crystallographer David P. Shoemaker (1920–1995), a student of Pauling’s at Caltech during the 1920s, describes Pauling’s unusual ability to impart his knowledge in the classroom and lecture theater with a flair for vivid showmanship, a trait that differentiated him from many other great scientists.
· From January 31 to February 1, 1960 Pauling became trapped on a ledge of a steep cliff on a dangerous, isolated stretch of rugged seashore near his Big Sur ranch, a disappearance that received wide press coverage. In “The Incident on the Cliff” (5 pp), a letter to his children describing his ordeal, he wrote, I am very sorry that I caused you and Mama so much anguish and concern.”
Part II. “Linus Pauling, The Science”
· In “The Scientific Contributions of Linus Pauling” (20 pp), an edited version of an appreciation that appeared in Biographical Memoirs of Fellows of the Royal Society, Jack Dunitz, professor emeritus of chemical crystallography at Zürich’s Eidgenössische Technische Hochschule (ETH), who worked as a postdoc with Pauling at Caltech between 1948 and 1954, reviews numerous highlights of Pauling’s long scientific career, including areas that have been slighted.
· In “Early Years of Physical Chemistry at Caltech” (10 pp), written while preparing an article for the Annual Review of Physics and Chemistry, Pauling describes his years as a graduate student and young professor and the atmosphere, the researchers, and the techniques (especially X-ray crystallography) that made the relatively small institution such an extraordinary place to practice science.
· Pauling’s first article on the chemical bond [12] is universally recognized as one of the most important papers in the history of chemistry. In “The Original Manuscript for The Nature of the Chemical Bond” (3 pp) he discusses its genesis and course of publication. After a typescript had been prepared, he tossed the handwritten manuscript, the first page of which is reproduced here, into the wastepaper basket, where it was retrieved by Ralph Hultgren, one of his graduate students, who retained it for 47 years!

Figure 3. Original handwritten manuscript of “The Nature of the Chemical Bond”, 1931 (p 108). From the Ava Helen and Linus Pauling Papers, Special Collections, Oregon State University.
· Although Nobel prizes are generally awarded for a single great discovery, Pauling, who had been considered a potential Nobelist since he was 30 years old, finally received the prize for chemistry in 1954 for what in effect was a career award. In “Modern Structural Chemistry: Nobel Lecture 1954” (8 pp), which has appeared elsewhere [13], he reviews his career.
· In “Pauling and Beadle” (8 pp), published in 1949 in Scientific American, science writer George Gray provides a summary of the state of molecular biology only a few years before the discovery of the structure of DNA and describes the partnership between Pauling and eminent geneticist George Beadle in their attempt to combine chemistry and biology to investigate the “molecules of life.”
· In “Sickle-Cell Anemia” (7 pp) Bruno Strasser discusses Pauling’s explanation of the properties of hemoglobin in terms of molecular structure and his founding of the field of molecular medicine.
· “How I Developed an Interest in the Question of the Nature of Life” (7 pp), is excerpted from what Pauling intended to be the first chapter of a book that he proposed to call The Nature of Life—Including My Life.
· Since Pauling and Robert B. Corey published their article on the alpha helix [14], the discovery and the priority for it have been the subject of controversy. In “The Discovery of the Alpha Helix” (9 pp), an essay published in 1982, Pauling offers his own memories of this seminal discovery.
· In “The Triple Helix” (19 pp) Tom Hager examines one of Pauling’s relatively rare failures. He discusses Pauling’s mistakes in method and approach in the race for the structure of DNA and the factors involved in the failure. During the early 1950s Pauling and most researchers, except for James D. Watson and Francis H. C. Crick, who received the 1962 Nobel Prize in Physiology or Medicine “for discoveries concerning the molecular structure of deoxyribonucleic acid,” believed that the genetic material was protein in nature rather than DNA.
· In the early 1960s Pauling and his postdoctoral fellow Emile Zuckerkandl developed the new field of molecular evolution, which has since grown and flourished. In “The Genesis of the Molecular Clock” (8 pp) Gregory J. Morgan examines their work and shows how it was related to the larger goals of Pauling’s life—to strengthen his argument against nuclear testing and increase the understanding of molecular disease.
· In “Orthomolecular Medicine Defined” (7 pp), which first appeared in 1968 [15], Pauling first introduced the word “orthomolecular,” meaning “the right molecules in the right amounts” because it was broader in scope than the term “megavitamin,” already used in a different context.
· In “There Will Always Be Something Interesting” (7 pp), an interview with reporter Neil A. Campbell conducted on October 22, 1980, Pauling reminisces on things that, in retrospect, seemed most important to him, especially the potential effects of a complete understanding of life at the molecular level. The 80-year-old scientist is much more realistic in his faith in progress than the enthusiastic youth who wrote “Children of the Dawn” in Part I above.
Part III. “Linus Pauling, The Peace Work”
· In 1945, following the dropping of the atomic bombs on Hiroshima and Nagasaki, Pauling spoke on nuclear fission before members of the Rotary Club in Hollywood. In “An Episode That Changed My Life” (3 pp), he relates how his wife then convinced him he should become knowledgeable about the nature of war and the need for peace, which caused him to devote at least half his efforts to world peace and world problems generally.
· In “The Ultimate Decision” (6 pp) Pauling, who had become a world leader in the peace movement, summarizes his advocacy of world government, civilian control of nuclear weapons, and international oversight of the development of new weaponry, and he emphasizes his route to a new world by letting citizens, informed by scientists, decide their fate rather than relying on government leaders.
· “Meet the Press” (7 pp) is the transcript of the May 18, 1958 program of the nationally televised public affairs show on which Pauling was aggressively “grilled” by host Lawrence Spivak and his panel of reporters to the outrage of Pauling and his wife. It shows how Pauling and his cause were negatively viewed by the mainstream press of the day.

Figure 4. Draft of a cable sent by Linus Pauling to President John F. Kennedy during the Cuban missile crisis, October 26, 1962 (p 218). From the Ava Helen and Linus Pauling Papers, Special Collections, Oregon State University.

Figure 5. Statement by Linus Pauling during his passport imbroglio, 1952 (p 153). From the Ava Helen and Linus Pauling Papers, Special Collections, Oregon State University.
· In “Science and Peace, Nobel Lecture 1963” (15 pp), which has appeared elsewhere [16], Pauling, who was surprised and pleased by the award, summarizes his thoughts after 15 years of working for peace.
· “Man—An Irrational Animal” (4 pp) is the text of a talk that Pauling presented at the Western Continental Congress for Peace in Mexico City on September 5, 1949, in which he argued that a one-world government based on scientific reasoning was the way to avoid a world-wide nuclear catastrophe.
· In “A World in Which Every Human Being Can Live a Good Life” (1 page, the shortest piece) Pauling proposes seven courses of action “for all nations and all people to cooperate in building a world free of war and militarism, a world based on rationality and ethics.”
Part IV. “Linus Pauling, Facets” (28 pp)
This section, admirably suited for browsing, consists of 42 quotations, many amusing and humorous, most of which are by Pauling, ranging from a single sentence to more than a page in length, that give insights into his character and personality and complete the portrait provided by the earlier pieces. Several examples, some of special interest to chemical educators, will suffice:
· “I also remember an incident in Amsterdam when you saved from injury or possible death a woman who was being dragged along the street alongside the wheel of a streetcar with which her clothes had become entangled” (David Shoemaker, letter to Pauling, 1982).
· “When I go to the market I always carry my slide rule to be sure the larger, bargain boxes of stuff are really bargains” (Pauling, 1966).
· “I read detective stories. I’ve soured on Agatha Christie. She lost me when she mentioned ‘foul odor of carbon monoxide,’ which is an odorless gas” (Pauling, 1987).
· “But in particular since my wife died, eight years ago. I don’t have anything to do now, except make discoveries and write papers” (Pauling, 1990).
· “I remember once when he was at dinner with some board of directors, and there were questions about virility. Pauling joked that he had not made love since 1955. Then he looked at his watch and said, ‘But it’s only 20:55 now.’” (Richard Hicks, 1991).
· [Rockefeller Foundation officer W. F.] “Loomis acknowledged that ‘Pauling certainly is imaginative, daring, and brilliant, but he has gone off the deep end in some cases…and his many stimulating pictures, models, etc., may be largely figments of his own imagination rather than lasting and sound science” (Lily E. Kay, 1993).
· “The photograph [in an eyepiece with a lens] was that of a beautiful girl, completely naked, standing on a large black rock in the middle of a rushing mountain stream. Pauling picked up the device and clapped it to his eye. ‘Hmmm,’ he said, ‘Basalt’” (Ken Hedberg, 1995).
· [Arthur Amos] “Noyes is reported to have said of his successor at Caltech, ‘Were all the rest of the chemistry department wiped away except [Pauling], it would still be one of the most important departments of chemistry in the world’” (Caltech professor Judith Goodstein, 1996).
· [During Pauling’s presentation of the electroneutrality principle] “Student: ‘Can you derive the principle?’ Pauling: ‘No. There is no derivation.’ Student: ‘Then how did you arrive at this principle?’ Pauling: ‘I made it up’” (Nobel laureate William Lipscomb, 1996).
· [After the attack on Pearl Harbor] “Linus and his wife Ava Helen had a Nisei gardener and they brought him and his family to live in their garage in an effort to keep him from being ‘detained.’ Later over the Paulings’ vigorous objections the Nisei family was forcibly removed and sent off to a detention camp” (Doug Strain, 2000).
· “I watch a moderate amount of television, mainly the news but, on occasion, if I can find an old Doris Day movie I watch it. My favorite is Lover Come Back, which has in it a character named Linus, who is described as the greatest chemist in the world….Finally, all the reporters come in to see the greatest chemist in the world’s new invention and there are trays of red and green cookies. It turns out that each one is the equivalent of a double martini” (Pauling, 1987).
· “A large beaker filled with what looked like water stood on the bench. Pauling entered, picked up a cube of sodium metal from a bottle, tossed it from hand to hand (done safely if your hands are dry) and warned of its violently explosive reaction with water. He then threw it into the beaker. As students cowered in fear of an explosion, he said nonchalantly ‘but its reaction with alcohol is much milder’” (Nobel laureate Max Perutz, 1998).
This attractive coffee-table book contains 84 photographs from Pauling’s earliest youth to his older years, many of which we had not seen previously, for example, one of Pauling in “drag” at an intrafraternity “smoker” in 1920 (p 30). It also includes drawings and reproductions of original manuscripts and typescripts by Pauling, A 9-page “Selected Bibliography” lists 101 of Pauling’s more than 1100 articles (1920–1994), 13 of Pauling’s 16 books, and 20 articles and books about Pauling, while a 7-page (double-column) index makes location of material easy.

Figure 6. Linus Pauling in “drag” (lower right) at an intra-fraternity smoker, 1920 (p 30). From the Ava Helen and Linus Pauling Papers, Special Collections, Oregon State University.
Taken together, the material in Mead and Hager’s collection illustrate Pauling’s weaknesses as well as strengths and portray the life and legacy of the most famous chemist of our time as an ambitious, complex, conflicted human being who spoke his own mind and lived a long and fruitful life on his own terms. In short, the book shows the complexities and inconsistencies of a creative, brilliant, and outspoken human being, who was neither saint nor sinner. We think that Pauling, who viewed himself as “a multifaceted crystal with many dimensions,” would be pleased with it. We recommend it heartily to both first-time readers about Pauling as well as to more knowledgeable scholars, who will benefit from the valuable unpublished source materials. It will also benefit general readers interested in the history of science, admirers of Pauling’s activism and peace work, as well as persons interested in chemistry, the history of chemistry and of science, nutrition and health, and peace. It is also an ideal complement to other collections of Pauling’s writings [17, 18].
References and Notes
1. For further details on Pauling’s work and life as well as the Pauling Centenary Celebration check out the Linus Pauling Centenary Celebration website: http://pauling.library.orst.edu (accessed July 2002). A short summary of his life is available online at http://www.accessexcellence.org/AB/BC/Linus_ Pauling.html (accessed Sept 2002). A collection of 46 of Pauling’s laboratory notebooks spanning 72 years is available online at: http://osulibrary.orst.edu/specialcollections/rnb/ index.html (accessed Sept 2002). A National Library of Medicine Profiles in Science digital exhibit dedicated to Pauling and containing more than 200 scanned letters, manuscripts, and photographs outlining Pauling’s biomedically-related work is available online at: http://profiles.nlm.nih.gov (accessed Sept 2002).
2. Wallace, J.; Mead, C. S., Eds. The Pauling Catalogue: Ava Helen & Linus Pauling Papers at Oregon State University; Kerr Library Special Collections; Oregon State University: Corvallis, OR, 1981.
3. Hager, T. Force of Nature: The Life of Linus Pauling; Simon & Schuster: New York, 1995; Kauffman, G. B.; Kauffman, L. M. Controversial Chemists. Angew. Chem. Int. Ed. Engl. 1997, 36, 1655–1657.
4. Hager, T. Linus Pauling and the Chemistry of Life; Oxford University Press: New York, Oxford, 1998; Kauffman, G. B.; Kauffman, L. M. Chem. Intelligencer 2000, 6 (1), 58–59.
5. White, F. M. Linus Pauling: Scientist and Crusader; Walker & Co.: New York, 1980.
6. Serafini, A. Linus Pauling: A Man and His Science; Paragon House: New York, 1989.
7. Linus Pauling: A Man of Intellect and Action; Cosmos Japan International: Tokyo, 1991.
8. Newton, D. E. Linus Pauling: Scientist and Advocate; Facts on File: New York, 1994; Kauffman, G. B.; Kauffman, L. M. J. Chem. Educ. 1997, 74, 380.
9. Goertzel, T.; Goertzel, B. Linus Pauling: A Life in Science and Politics; Basic Books: New York, 1995; Kauffman, G. B.; Kauffman, L. M. Controversial Chemists. Angew. Chem. Int. Ed. Engl. 1997, 36, 1655–1657.
10. French, A. P., Ed. Einstein: A Centenary Volume; Harvard University Press: Cambridge, MA, 1980.
11. Kennedy, P. J., Ed. Niels Bohr: A Centenary Volume; Harvard University Press: Cambridge, MA, 1985.
12. Pauling, L. The Nature of the Chemical Bond. Application of Results Obtained from the Quantum Mechanics and from a Theory of Paramagnetic Susceptibility to the Structure of Molecules. J. Am. Chem. Soc. 1931, 53, 1367–1400.
13. In Nobel Foundation. Nobel Lectures Including Presentation Speeches and Laureates’ Biographies; Chemistry 1942–1962; Elsevier Publishing Co.: Amsterdam-London-New York, 1964; pp 425–437; for G. Hägg’s presentation speech see: http://www.nobel.se/chemistry/laureates/1954/press.html (accessed Sept 2002) and for Pauling’s Nobel lecture, “Modern Structural Chemistry,” see: http://www. nobel.se/chemistry/laureates/1954/pauling-lecture.html (accessed Sept 2002).
14. Pauling, L.; Corey, R. B. Two Hydrogen-bonded Spiral Configurations of the Polypeptide Chain. J. Am. Chem. Soc. 1950, 72, 5349.
15. Pauling, L. Orthomolecular Psychiatry: Varying the concentrations of substances normally present in the human body may control mental disease. Science 1968, 160, 265–271.
16. For Gunnar Jahn’s presentation speech visit: http://www.nobel.se/peace/laureates/1962/press.html (accessed Sept 2002). For Pauling’s Nobel lecture, “Science and Peace,” see: http://www.nobel.se/peace/laureates/1962/pauling-lecture .html (accessed Sept 2002).
17. Marinacci, B.,Ed. Linus Pauling in His Own Words: Selections from His Writings, Speeches, and Interviews; Simon & Schuster: New York, 1995; Kauffman, G. B.; Kauffman, L. M. Controversial Chemists. Angew. Chem. Int. Ed. Engl. 1997, 36, 1655–1657.
18. Linus Pauling on Peace: A Scientist Speaks Out on Humanism and World Survival; Selected and Edited by Barbara Marinacci and Ramesh Krishnamurthy; Rising Sun Press: Los Altos, CA, 1998; Kauffman, G. B.; Kauffman, L. M. Chem. Educator 1999, 4 (5), 198–199; DOI 10.1007/s0008979900332a.
George B. Kauffman and Laurie M. Kauffman,
California State University, Fresno,
S1430-4171(02)05606-X, 10.1007/s00897020606a
Creative Evolutionary Systems. Peter J. Bentley and David W. Corne, editors. Academic Press: San Diego, Morgan Kaufmann: San Francisco. £43.95. ISBN 1-55860-673-4.

Evolutionary Computation (EC) is a technique, which is rapidly developing a momentum of its own. A set of computational methods within the field of Artificial Intelligence, the development of Evolutionary Computation has been driven largely from within computer science, but the method has expanded to become an increasingly powerful way of solving scientific problems. As this volume shows, it can also be the source of much entertainment and fascination, and even of work by computers that is apparently creative and artistic.
EC programs work through processes that are akin to natural evolution. Some practitioners have argued in the past that the origins in natural processes of Genetic Algorithms and other EC methods makes such techniques special, somehow inherently superior to the more abstract methods devised without reference to the way nature solves problems. This view is no longer widely held, and it has always begged the question of how closely an algorithm must resemble a natural process before the algorithm becomes able to outperform “synthetic” methods.
When looked at in this way, it is easy to appreciate that evolutionary methods are probably not endowed with magical powers; however, while there is little to suggest that these methods are somehow born superior to all others, there is no doubt that they are very different from “conventional” methods of tackling problems. This special nature may encourage us to consider problems from a different angle, and a new viewpoint does often permit the discovery of different and interesting solutions.
When solving a problem, scientists may know, or be able to devise, some suitable analytical method that will deliver the “right” answer with acceptable uncertainty and in a tolerable period of time. Often, though, such an analytical approach cannot be found. If this is the case, it is common to start by making an initial guess at a solution and then trying to improve it iteratively until a solution of acceptable quality is located, or until we become bored in the attempt. This is the kind of approach used in successive approximation or in simulated annealing, for example.
EC programs are radically different from such a single-solution approach: they start not from one trial solution, but from a multitude, a so-called population of solutions. This entire collection is worked upon and refined simultaneously, as the algorithm tries to improve the quality of the whole population, with the aim of gradually moving the population toward good, or optimum, solutions. If it is to succeed, the algorithm must have some means by which it can recognize those solutions that are of better quality than their fellows. In addition, a method is needed by which it can alter solutions within the population in a valid way so that new—potentially superior—solutions can be created.
EC methods, falling within the regime of intelligent algorithms, are sometimes viewed as—if not magic—at least possessing in some measure the ability to drive solutions in a sentient way toward the optimum, but in no sense do they do this. They are no better than other algorithms at knowing where optimum solutions are located, but the evolutionary forces, which they encapsulate, do push the overall quality of the population towards better solutions, and in the long term they often locate solutions of impressive quality.
Dave Corne and Peter Bentley have been active in EC for many years, and have quite eclectic interests in the field. Although EC methods have wide application in chemistry and the other sciences, the focus of this book is, as the title suggests, very much on the use of EC for creative purposes, rather than for solving scientific problems. It is nonetheless fascinating for all that, (indeed one might argue that, by being less closely tied to an academic imperative than most other books in the field, the book is free to become even more intriguing). Nevertheless, a few scientific applications are included for those who wish to be reminded that the algorithms are more than mere toys.
Many of the illustrations in the book and on the Web of the applications of EC are striking, even beautiful.

Skaters, Steven Rooke, 1997.

In the beginning, Steven Rooke, 1996.

An image by Karl Sims, 1991.
The authors of the software described in the book sometimes have set out deliberately to produce attractive or dramatic images, but, once started, the programs take over their own evolution. This leads some authors to conclude that the computer is itself being creative. Whether the computers are—or even can be—knowingly creative depends upon the position of the reader, and what one means by creativity. There is little doubt, however, that much of what is produced by EC is more artistically palatable than bisected sheep or much other modern art, and a good deal cheaper to acquire.
The book begins with a detailed look at the principles of creative evolutionary systems. Running to 75 pages, this introduction by Bentley and Corne is lucid and straightforward and will be familiar ground for any reader who has come across Genetic Algorithms before; however, the examples chosen to illustrate the discussion are drawn from design, rather than computer science, in keeping with the theme of the book. There is an extensive list of references (12 pages), which provides useful background material for those new to the field.
Genetic Algorithms, Genetic Programming, Evolution Strategies and Evolutionary Programming are all discussed, with an outline of how such techniques may be applied to creative studies.
An interesting, though perhaps unnecessarily short, section is included concerning how to encourage algorithms to be creative. This may sound as though it would be of interest only to artists or computer nerds, but Parmee’s work in engineering shows how constructing an algorithm that is in some sense creative can be of value even in an engineering or scientific application. The key is often the removal of the constraints that would otherwise confine solutions to lie within strict, and on the face of it reasonable, limits. The looser the constraints, the more freedom the algorithm has to evolve new (and potentially attractive) solutions.
This introduction is followed by sections that discuss Evolutionary Creativity, Evolutionary Music, Creative Evolutionary Design, Evolutionary Art, and Evolutionary Innovation. Several of the authors, including David Andre, Forrest Bennett III, Peter Elliott, Peter Hancock, and (the prolific) John Koza, will be well known to those working in the field.
The range of applications is considerable. To those coming to Evolutionary Computation for the first time, some of the applications may seem trivial, or difficult to fathom. Helen Jackson’s discussion of the use of an L-system GP to evolve architectural forms, for example, may strike some as having only peripheral relevance to the construction of real buildings, while the chapter on “Discovering novel fighter combat maneuvers: Simulating Test Pilot Creativity” by Smith et al, while apparently belonging here on the basis of its title, seems to slip into the book by only the narrowest of margins. One wonders also at the value of some of the other studies described in the book. This, however, is perhaps to try to read more depth into the chapters than is intended, since the thrust of the book is more into aesthetics than utility. In reality every chapter is worth reading, and many merit a second look.
This emphasis on the creative aspect of the algorithms does not mean that “real” applications are given entirely a back seat. Julian Miller and co-workers produce an interesting discussion of the evolution of new electrical circuits and Paul Brown’s work on Cellular Automata will strike a chord with anyone who has played with the game of Life. (This struck a particular resonance with me. In the mid-1960s I was a terror-struck mathematics student interviewed for a place at Cambridge University by the eccentric and remarkable John Horton Conway, the creator of the Game of Life. He was, without doubt, the most intimidating man I have ever met, exploding with disconnected mathematical ideas and questions of unfathomable obscurity over an interview which lasted two hours.)
Peter von Buelow introduces another of the more down-to-earth applications in the book, describing how one might use AI in the design of architectural structures, while Shail Patel at Unilever research and co-workers describe evolution of bacterial peptides. Steven Rooke’s and Karl Sims’ striking images, which are used to illustrate this review, show the ability of GP to produce visually arresting images.
A CD accompanies the book, but this was something of a disappointment. The CD relied too heavily on links to the Web, which one might have discovered for oneself, and included too few genuinely flexible applications with source code which could be adapted for one’s own enjoyment. Unfortunately, this difference in value between a CD and the book, which it accompanies, seems common; it certainly is not limited to books in computer-related fields. Too frequently CDs seem to be an afterthought rather than something that should be given as much care and time as the text itself.
Perhaps the problem is that, although one is buying both the book and CD, the accompanying CD is often included “free.” Accordingly, it might be in the publisher’s mind that the CD does not need to be of such a high standard. Perhaps publishers should try advertising a CD with a free book attached—that might focus the attentions of the authors on the CD and perhaps raise its quality. The contributors to this book may be concerned that not too much of their inventive software should be made widely available, but if that is the case, why offer a CD?
The CD is a disappointment, but little else about this book is. Its title makes it clear that the emphasis is on creativity, but one might almost suggest that it is about having fun. Few chemists will buy this book in order to find out how to apply Evolutionary Algorithms to chemistry, there are better books around for that purpose. Many, though, might wish to have a copy to see some of the fascinating ways in which these valuable and entertaining algorithms can be a source of fun and beauty.
Hugh Cartwright,
Physical and Theoretical Chemistry Laboratory,
Oxford University, U.K., Hugh.Cartwright@chem.ox.ac.uk
S1430-4171(02)05607-9, 10.1007/s00897020607a
Electroanalytical Methods: Guide to Experiments and Applications. F. Scholz, Editor. Springer-Verlag: Berlin, 2002. 331 pp. 100 figures, 31 tables. £49.00 (€69.95 + VAT) ISBN: 3-540-42229-3 (hardcover).
Electrochemistry is a diverse subject practiced by a multitude of workers in different branches of science, as noted in the preface of this monograph. Its employment and attractiveness stems from its ability to provide both fundamental thermodynamic parameters and reaction kinetics data quickly, often simultaneously, and with little cost to the experimentalist. In other applications, the ability to detect a single substance electrochemically in the presence of other similar substances, and under a plethora of conditions, is an innovative art in itself. This last application, together with research into batteries and fuel cells, brings a large investment into electrochemical research.
Not surprisingly, the last few years have seen a plethora of texts devoted to both the basics of and current research trends in all parts of electrochemistry, most notably Wang’s Analytical Electrochemistry (see, for example, N. S. Lawrence, Chem. Educator [Online], 2002, 7, 180–181, S1430-4171(02)03566-9; DOI 10.1007/s00897020566a), the new edition Bard and Faulkner, Girault’s text (in French), and Wiley’s 12-volume Encyclopaedia of Electrochemistry. Given the competition, students of electrochemistry might be forgiven for being skeptical about the aim of the book as becoming a “bench book [to be] used in the laboratory.” However, Fritz Scholz and his twelve-strong team (selected, with the exception of Alan Bond, from a European background, and including the editors-in-chief of both Electrochemistry Communications and Journal of Solid State Electrochemistry, and a senior editor of the latter journal) are to be congratulated on the production of a highly readable and well-structured book.
The book is structured into four parts after a fairly comprehensive glossary. The first part deals with the fundamentals of electrochemical science, starting with a brief discussion on the structure of the double layer. The second chapter in this section introduces the reader to electrode potentials and their use in the extraction of thermodynamic data, and it is followed by a very good account of the kinetics of the electron-transfer process.
The second part of the book introduces nine techniques used in electroanalytical research. These chapters all extensively reference typical applications and, in some cases, are written by specialists in the field, such as Lovrić on square-wave voltammetry, Compton on spectroelectrochemistry, and Scholz on solid-state electrochemistry.
The first chapter in this section is on cyclic voltammetry. This is perhaps one of the most comprehensive chapters in the entire book. Although (given the nature of the audience), the treatment avoids rigorous mathematics, it nevertheless provides a thorough discussion of the understanding and use of cyclic voltammetry in a variety of systems. The second chapter examines step and pulse techniques, and it is followed by a chapter covering the process that is perhaps the one most widely used by applied electrochemists, square-wave voltammetry. Lovrić provides an excellent account of the principles and general use of square-wave voltammetry, and he treats the mathematically inclined readers to a 66-equation feast in the appendix—the only chapter in the book with one. This chapter is followed by a very lucid account of chronocoulometry and, subsequently, a rigorous summary of impedance spectroscopy.
The sixth chapter in Part II of the book provides an excellent discussion of spectroelectrochemistry in the UV/visible/near-infrared region. This is perhaps one of the highlights of the text, illustrating some very innovative approaches in the pursuit of electrochemical reaction mechanistic and kinetic data. Of particular interest is Table II.6.1, which presents a brief discussion of various spectroelectrochemical techniques. Chapter 7 deals with the one of the most well-known electrochemical techniques employed in the analysis of heavy metals, stripping voltammetry. In his review, Lovrić provides an enjoyable account of both old and new applications of this traditional technique. This is followed by a brief summary of solid-state electrochemistry.
In contrast to Bruce’s book on the subject, Scholz and Fiedler write exclusively about the voltammetry of microparticles, a technique pioneered by Scholz over the last thirteen years. No electrochemistry text would be complete without a discussion on potentiometry, a feat Kahlert manages in 18 sides.
The third part of the book covers experimental details, and includes two very good discussions on working electrodes and electrolytes by Kormorsky-Lovrić, an excellent account on reference electrodes, and finally a brief note on experimental set up. This whole section is very well written, complements the book, and is of interest to all practicing electrochemists.
Part IV of this book gives the reader an opportunity to learn about the wider electrochemistry community. First, it provides a compilation of the most important breakthroughs in electrochemical science, from Galvani’s first experiments on frogs to Bard’s development of scanning electrochemical microscopy. Second, it provides an account of various sources on information of electrochemistry, making particular use of the World Wide Web, thus providing a good conclusion to the book.
The book is very well laid out, and easy to read. Although the publishers provide a list of errata, several typographical errors remain. All in all, I think this is a very well written book, and will be useful for both undergraduates and practicing electrochemists alike, both in and outside the laboratory.
Jay D. Wadhawan,
Physical and Theoretical Chemistry Laboratory,
Oxford University, jay@physchem.ox.ac.uk.
S1430-4171(02)05608-8, 10.1007/s00897020608a
Inorganic Syntheses, Vol. 33. Dimitri Coucouvanis, Editor-in-Chief. John Wiley & Sons: New York, 2002. xxiv + 276 pp. hardcover. 15.5 ´ 23.5 cm. $90.00. ISBN 0-471-20825-6.
This popular series, designed "to provide all users of inorganic substances with detailed and foolproof procedures for the preparation of important and timely compounds," plays an extremely important role in the burgeoning literature of inorganic chemistry. Because each preparation is experimentally checked independently for reproducibility and yield in a laboratory other than that of the submitters and the sources of reagents are given, Inorganic Syntheses is recognized worldwide as the primary source for the preparation of a multitude of useful inorganic substances. The series is a truly international undertaking; this volume includes submitters and checkers from academic and industrial laboratories in 16 countries. Furthermore, because it includes not only substances traditionally considered inorganic but also those of concern to workers in other fields (in this volume, enzymology, bioorganic, bioinorganic, and organometallic chemistry, photochemistry, and materials science), it is of interest to the entire scientific community.
This latest volume in the series emphasizes the new directions in which inorganic chemistry has advanced during the past two decades. Many of the new areas of interest involve complexity in design as well as molecules containing specific functional groups. These characteristics, inspired either by metalloenzymology or the need for new materials with specific properties and functions, are essential features of synthetic “supramolecules” or clusters.
Supramolecular methodology and syntheses of supramolecular assemblies have not been previously emphasized in Inorganic Syntheses for a number of reasons—the difficulty inherent in the synthesis of multi-unit assemblies, the possible lack of general interest for specific supramolecules, and the lack of a clear demonstration of utility and function for many of these molecules. Since 1987, however, when Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen received the Nobel Prize in chemistry “for their development and use of molecules with structure-specific interactions of high selectivity,” the need for synthetic methodology and actual examples of supramolecular assemblies in the series became evident.
The volume under review includes procedures for preparing 193 individual substances, arranged in 42 numbered sections in four chapters. Chapter 1, "Syntheses of Selected Supramolecules" (54 compounds), includes procedures and methodology for the synthesis of molecules that can serve as magnetic building blocks, light-gathering units, and ditopic activators or receptors that may inspire similar syntheses of new materials using similar methodologies. One of the syntheses uses the “complexes as metals/complexes as ligands” strategy—a general method for the synthesis of polynuclear metal complexes—to prepare a luminescent complex. Another features the first well-characterized multi-iron sandwich compounds prepared by rational synthesis from lacunary or defect species, while one involves metallacrowns—a new class of molecular recognition agents that selectively bind cations and anions in structures similar to organic crown ethers.
In the synthesis of new coordination compounds, both classic and supramolecular, reagents that serve as convenient sources of metal ions or metal-containing groups are of utmost importance. A number of such molecules useful as reagents or building blocks are included in Chapters 2 and 4. Chapter 2, "Useful Reagents and Ligands" (46 compounds), includes precursors for a variety of nonaqueous synthetic applications, sources of metal ions soluble in nonaqueous solvents that possess very weakly or noncoordinating redox-stable anions, polynuclear iron complexes, tetrathiorhenates—complexes that are unique in their high reactivity toward unsaturated organic compounds, and polythioether macrocycles or “thiacrowns,” which serve as excellent ligands for transition metals.
Because low temperature solid-state synthesis is a technique that shows great promise for the synthesis of materials with unusual and interesting properties, it was the subject of Volume 30 of this series (Nonmolecular Solids, Donald W. Murphy and Leonard V. Interrante, Editors-in-Chief, 1995). However, since interest has continued in solid-state materials, especially those obtained by relatively low temperature procedures, another series of such procedures and syntheses are included in Chapter 3, "Solid-State Materials and Clusters" (41 compounds). The chapter includes syntheses of solid-state compounds by molten salt fluxes, hydrothermal crystallization used when compounds are difficult or impossible to produce by other synthetic methods, open framework solids, and sulfur-bridged cubane type mixed-metal clusters.
Chapter 4, "Compounds of General Interest" (52 compounds), includes an improved synthesis of a charge transfer sensitizer for dye-sensitized nanocrystalline titanium(IV) oxide solar cells as well a preparation of a luminescent rhenium(I) tricarbonyl complex that has found application as a molecular probe and as photosensitizer for solar energy conversion. Most of the syntheses in the volume involve extremely complex equipment and time-consuming procedures. However, this final chapter contains a few relatively simple compounds such as tetrasulfur tetranitride and arsenic(III) bromide, whose preparation may be suitable for undergraduate inorganic laboratory courses, although the former is potentially explosive and the latter is toxic.
Many of the preparations involve starting materials or products that are flammable; lachrymatory; irritating; noxious; air-, moisture-, or temperature-sensitive; toxic; pyrophoric; corrosive; carcinogenic; teratogenic; or potentially explosive, but any hazards are specifically identified, and safety precautions are emphasized. The volume is replete with detailed diagrams of the frequently complicated apparatus or equipment required for many of the preparations, any of which pose a real challenge for even the most experienced students.
The current emphasis on supramolecules and clusters in both academic and industrial chemistry make this volume, which maintains the high standards set by its predecessors, particularly timely and useful.
George B. Kauffman,
California State University, Fresno, georgek@csufresno.edu
S1430-4171(02)05609-7, 10.1007/s00897020609a
Organic Chemistry Science Tutorials. M. C. H. Multimedia, Inc. Compact Disc. Order from: M. C. H. Multimedia, Inc., 1-888-390-7947, Web site: mchmultimedia.com; $79.99 (student price: $39.99).
There is now a wealth of instructional compact discs available that cover basic topics in organic chemistry, many of which are supplementary materials for courses or textbooks. These CDs aim to provide a visually based resource to help students understand some of the more difficult concepts in organic chemistry. Additionally, they tend to engage technology-minded students and provide another venue, outside the classroom, for students to learn.
M. C. H. Multimedia's compact disc on organic chemistry is design to help students learn organic chemistry in an engaging visual fashion. The CD is organized like many textbooks, with the main organization being centered around the functional groups. In addition to basic functional-group categories there are sections on biochemistry, nomenclature, acid–base reactions, as well as stereochemistry and isomerism.
Installation of the CD and this software did not proceed smoothly. The CD was unreadable by both PCs and Macintosh computers using a variety of different operating systems. After a number of futile attempts to load this software, I decided to try M. C. H. Multiamedia technical support. Calling the toll-free phone number that was provided, the technical staff provided me with a new code that supposedly would allow the software to function. This failed to work, and eventually M. C. H. Multimedia sent me another copy of the CD. The installation proceeded more smoothly the second time but I still had to call technical support several more times because the unlock key provided with the software would not allow me to access the main startup screen.
The functional group organization of this software is apparent upon accessing the main screen or the top level of the CD. The first of the fifteen main categories on the startup screen is called "Quick Tour." This choice from the main menu allows the user to become familiar with some of the basic content of the software, and it orients the user to most of the navigational features.
The Quick Tour feature explains how to navigate from tutorial to tutorial using the table of contents, and it also shows the user how to search a tutorial using key word searches. Other features, such as how to get back to the main page or how to access the “blank notebook” are also shown. This first tutorial runs more like a movie because the hypertext links and interaction buttons for the program are all disabled. In some cases the images used in the explanations in this CD seem more geared to physical chemistry than to organic chemistry. This might be, in large part, due to the fact that interactive programs for introductory and physical chemistry are included on the same compact disc.
Some features of the CD that were helpful included the red checkmark that appears by the topics so that the user knows how much material has been covered in each tutorial. Additionally, there is a quiz associated with each tutorial, and the quick tour explains how to access the quizzes for each different topic. Another nice feature of this program is the ability to turn on or off the voice that narrates all of the pop-up screens. Yellow text boxes are used to explain features during the Quick Tour, but occasionally the text in these boxes was not centered, or, worse, extended outside the yellow box.
The tutorials themselves contained some mistakes that detract from this CD. The tutorial entitled "Overview of Organic Chemistry," which is the first tutorial that students might look at after the Quick Tour, had several spelling errors. For instance, the title to the section "Aldehydes and Ketones" was spelled "Adlehydes and Ketones." Further, the narration voice for the tutorials, with the exception of the Quick Tour, is a little annoying, including some mispronunciations. In addition to these technical and grammatical mistakes there were some cases where clicking on a link gave no information on the desired topic. For instance, in the "Overview of Organic Chemistry," clicking on Organic Molecules gave only information on how to use the program, information that would seem more useful in the Quick Tour section. Other issues that might be hard for students are the wide variety of structure styles and the sometimes bizarre mix of condensed and line-angle structures shown in the same scheme. This is compounded by the fact that little care has been given to ensuring that bond lengths are the same for C–C bonds, giving structures a less that neat appearance in some cases.
Perhaps more serious, however, are errors with regards to content. In the acids and bases section too much emphasis is placed on calculating the exact concentration of weak acids and bases, which seems more appropriate for a student in introductory chemistry. The program prompts students to examine the pKa of different compounds, specifically asking the students to look at the pKa of water, which interestingly is reported as between 19 and 20. Examination of the other tutorials shows similar types of mistakes.
Some of the reaction animations might be useful to students who need to visualize each individual step, but it also would be nice if the arrow-pushing convention was included as well. Other reaction animations are confusing even to people who have thought about organic reactions for a considerable amount of time, and it must be equally or more confusing for students.
There are a number of choices for instructional compact discs that cover organic chemistry, many of which have successfully provided a visually based resource to help students understand organic chemistry. M. C. H. Multimedia's compact disc on organic chemistry is designed to help students learn organic chemistry in an engaging visual fashion, but has not accomplished this as well as some other products that are currently available on the market. With proper editing this CD might compete successfully with other organic chemistry CDs, but the version that was reviewed still needs some polishing.
Jeff Willemsen
Willamette University, jwillems@willamette.edu
S1430-4171(02)05610-6, 10.1007/s00897020610a