 The
Chemical Educator, Vol.
8, No.2, Media Reviews, © 2003 The Chemical Educator
The
Chemical Educator, Vol.
8, No.2, Media Reviews, © 2003 The Chemical Educator
Media Review
 The
Chemical Educator, Vol.
8, No.2, Media Reviews, © 2003 The Chemical Educator
The
Chemical Educator, Vol.
8, No.2, Media Reviews, © 2003 The Chemical Educator
Media Review
Survival Guide for Physical Chemistry. Michelle Francl. Physics Curriculum and Instruction: Lakeville, MN, 2001. vi + 136 pp, paperback. $17.95. ISBN 0-9713134-0-7.

This book is intended for undergraduate students studying physical chemistry as part of a major sequence in chemistry. There are four extended chapters—“Lecture,” “Guerrilla Math,” “Beyond Pencil and Paper,” and “The Write Stuff.” There are concise, well-chosen “more information” reading lists and an index.
Chapter 1 should be required reading for all chemistry students. There is much more than just the “lecture” of the chapter title. There is advice on how to prepare before each lecture, how to learn effectively in class and afterwards, collaborative work in groups, problem-solving techniques, and examination preparation and technique. Professor Francl’s style is easy to read, almost conversational in tone, and with good use of humor. I particularly liked the section on how students should treat their professor (lecturer) as ordinary human beings, who are entitled to “time off,” time with family, and even time to recover from illness!
The major part of the book is Chapter 2. The author writes: “The various sections of this chapter are designed to remind you of things you already know…and to add to your mathematical armory,” things which students should have picked up in “the two or three semesters of calculus that are typically required for p-chem.” This is more a compilation of useful mathematical methods, rather than remedial help for struggling students, who will find a useful bibliography of references at the end of every chapter. This chapter is very comprehensive: everything that should be included, was included. I did not expect the formula for the fourth-order Runge-Kutta method for numerically solving first-order differential equations, but it was there too. Because of the subject matter of the book, this is the only chapter with problems. Fully worked answers are given in an appendix.
Chapter 3 is about using computers to analyze numerical data. The aim here is for students to be aware of what computer packages can do—if one package can, for example, fit data, then most packages can probably do it too. There are some examples of the use of Basic, Fortran and (Excel) spreadsheets, but most of the examples are drawn from the use of Mathematica. The reading list for this chapter is somewhat limited. Well-chosen books on calculus, applied mathematics, programming, and Mathematica are listed, but one or two references on the use of MathCAD and spreadsheets would also be helpful.
Chapter 4 covers how to write laboratory notebooks and reports. This is a good introduction to academic writing and covers all the important technical aspects, for example, the format of a report, advice that all spectra should be included in the laboratory notebook, and even what type of notebook should be purchased.
In summary, this is an excellent book. To paraphrase the author, this is the one book an undergraduate student would want when finishing that p-chem paper at 1 a.m.!
References and Notes
1. Markow, P. G. J. Chem. Educ. 1988, 65, 346.
2. Rickey, D.; Stacy, A. M., J. Chem. Educ. 2000, 77, 915.
Kieran Fergus LIM (![]() )
)
School of Biological and Chemical Sciences, Deakin University, Australia, lim@deakin.edu.au
S1430-4171(03)02679-2, 10.1333/s00897030679a
Oxygen: A Play in Two Acts. By Carl Djerassi and Roald Hoffmann. Wiley-VCH: Weinheim, Germany; New York, NY, 2001. Illustrations. vii + 119 pp; 13.0 ´ 20.9 cm.; paperback. $14.95; £9.99; ISBN 3-527-30413-4.

Carl Djerassi [1], the Stanford University professor emeritus of chemistry who received the National Medal of Science (1973) for the first oral contraceptive and the National Medal of Technology (1993) for promoting new approaches to insect control, has undertaken a highly successful career in creative writing, including a tetralogy of novels that exemplify what he calls “science-in-fiction” to differentiate it from the better known science fiction. In 1998 he embarked on a projected trilogy of plays to explore a genre that he calls “science-in-theater.” His first play in this genre, An Immaculate Misconception [2a], dealt with ICSI—intracytoplasmic sperm injection. Our review [2b] discusses this play as well as many of Djerassi’s other works in creative writing.
Although we have witnessed a spate of plays with scientific themes recently (more than 20 plays in the last five years, the most celebrated of which is Michael Frayn’s 2000 Tony-winning Copenhagen [1998], which reenacts Werner Heisenberg’s 1941 visit to his mentor and friend Niels Bohr in Nazi-occupied Denmark), plays involving science or scientists have a long history [3, 4]. We can trace the genre as far back as Christopher Marlowe’s Dr. Faustus (1604) about a scientist who strikes a bargain with the Devil and Ben Jonson’s The Alchemist (1610), lampooning the venerable pseudoscience that was the ancestor of our own chemistry. In recent years “science plays” have centered on physics, possibly because of its involvement with the nuclear bomb.
Djerassi’s second play, coauthored with 1981 Nobel chemistry laureate Roald Hoffmann [5], the Frank H.T. Rhodes Professor of Humane Letters and Professor of Chemistry at Cornell University, not only deviates from the current preoccupation with physics but also has the distinction of being the first “science play” written by two practicing scientists. Like Djerassi, Hoffmann has also pursued a career in literature, being the presenter of the PBS television series The World of Chemistry [6], author of numerous essays, five books of poetry [7–11], and three nonfiction books dealing with the relationship of chemistry to society [12–14].
Ten readings and staged workshop productions of Oxygen, the usual stages in the development of a play, took place from October 6, 1999 to January 12, 2001. In celebration of the centenary of the first Nobel prizes, its world premiere (eight performances by the San Diego Repertory Theatre, directed by Bryan Bevell) took place at the Lyceum Theatre in San Diego, CA from April 2 through April 7, 2001 concurrently with the 221stNational Meeting of the American Chemical Society. In an unusual twist the play in book format appeared shortly before the world premiere (According to Djerassi, the play can be understood and enjoyed by reading as well as by viewing).
Through April 2003 readings took place at the Hörsaal der Charité, Berlin; the New York Academy of Sciences; Miami University, Middletown, OH; Trinity College, Hartford, CT; Kenyon College, Gambier, OH; and the City University of New York Graduate Center. It was broadcast on December 2, 2001 by BBC Radio on its World Service as “Play of the Week” and on December 12, 2001 by Westdeutscher Rundfunk (WDR Radio 3) in a German adaptation.
The German language premiere, directed by Isabella Gregor, took place at the Mainfranken Kammerspielen Theater, Würzberg on September 23–29, 2001, followed by a regular run during the 2001/2002 season. Additional staged performances took place at the Royal Institution of Great Britain, London (October 27–29, 2001); Fletcher Opera Theatre, Raleigh, NC in connection with the Sigma Xi Annual Meeting (November 8 and 10, 2001); Riverside Studio Theatre, London (November 14–December 1, 2001); Deutsches Museum Munich (November 23–25, 2001); “Erholungshaus-Bayer,” Leverkusen (February 24, 2002); Chemistry Department, University of New Hampshire, Durham, NH (May 11, 2002); 350th Anniversary Celebration of Leopoldina, Halle (June 20, 2002); Michigan State University, East Lansing, MI (September 27–29, 2002); Ohio State University, Columbus, OH (February 26–March 8, 2003); and the University of Wisconsin Theatre, Madison, WI (March 28–April 8, 2003).
Because Djerassi was at Stanford and Hoffmann was at Cornell, the pair first collaborated in New York and San Francisco hotel rooms as well as during as a ten-day period together in London. They then worked by exchanging about 2000 emails, in which they corrected the manuscript by using the tracking mode so that each could clearly see each round of changes. In Djerassi’s words, “Without email, it probably would not have been realistic to do this” [15].
Controversies arose between the two, as would be expected in dealings with persons with strong personalities. On three occasions, they referred the matter to three different dramaturgs, who acted as counselors—Alan Drury of BBC Radio; Ed Cohen, former associate director of the Jewish Repertory Theater, New York; and Ed Hastings, former artistic director of the American Conservatory Theater, San Francisco. At times when they still could not agree, they used Djerassi’s wife—writer and poet Diane Middlebrook—as a referee, a fortunate choice since the women in the play have central roles. The end result was, in Djerassi’s words, “an absolute collaboration. We are one person, ‘Carl Hoffmann and Roald Djerassi’” [15].
The fictional premise of the play is that to celebrate the 100th anniversary of the first Nobel Prizes, the Nobel Foundation decides to award a “retro-Nobel” Prize for great discoveries that preceded the establishment of the prizes in 1901. The Chemistry Committee quickly decides to honor the discovery of oxygen, the gas that ushered in the Chemical Revolution. But thorny questions immediately surface: “What is discovery? Why is it so important to be first?” (These are the first two sentences of the play’s foreword, p V). Ask any freshman chemistry student who discovered oxygen, and the inevitable response would be to name the English Unitarian clergyman and chemist Joseph Priestley (1733–1804) [16].
Priestley prepared what he called “dephlogisticated air” by heating mercurius calcinatus (mercuric oxide) with his burning lens on August 1, 1774 and published his results that same year [17]. However, the Swedish apothecary and chemist Carl Wilhelm Scheele (1742–1786), whose name is unfamiliar even to most practicing chemists, prepared what he called Feuerluft (fire air) in 1771 and 1772, but the book in German describing his discovery did not appear until 1777; its publication was held up partly by Torbern Bergman’s delay in delivering the preface [18]. The third candidate for the discovery is Antoine-Laurent Lavoisier (1743–1794), the French chemist, tax collector, economist, and public servant—the founder of modern chemistry, who explained the true nature of combustion, rusting, and animal respiration as well as the central role of oxygen (the name which he mistakenly gave the gas from the Greek meaning “acid former”) before his life was cut short on the guillotine on May 8, 1794. In a visit to Paris in October 1774 Priestley told Lavoisier of his discovery, as did Scheele in a letter that same year, to which Lavoisier never replied. Both Priestley and Scheele interpreted their results in terms of the now discredited phlogiston theory, which Lavoisier successfully overthrew.
According to Djerassi,
You have three people: one who did it first, Scheele; one who published it first, Priestley; and one who understood it first, Lavoisier. So, who is, in fact, the discoverer of oxygen? That’s the basic problem we have in contemporary science all the time…. What happened in 1777 can really be translated into 2001, because it is the same thing [15].
Actually, the problem exists in society in general. For example, Columbus’ position as the discoverer of America has been questioned. After all, like Priestley and Scheele, he did not understand his discovery. He thought that he had landed in the East Indies and that the island of Cuba was Japan (Cipango).
Djerassi and Hoffmann’s play attempts to answer the questions:
How does Lavoisier deal with the Priestley and Scheele discoveries? Does he give the discoverers their due credit? And what is discovery after all? Does it matter if you do not fully understand what you have found? Or if you do not let the world know? (p VI).
The action of the play takes place in Stockholm and shifts back and forth from 1777, when Swedish King Gustav III of Giuseppe Verdi’s “Un Ballo in Maschera” fame is supposedly offering a prize for the discovery of oxygen, and 2001, with the same actors and actresses appearing in both dual and parallel time frames, underscoring the fact that time has not changed human beings—their motivations, their emotions; desire for recognition, power, and financial rewards; concern with reputation and awards; and their social institutions—in this case, scientific behavior and culture. The ethical issues of ambition, competition, discovery, and priority addressed in Oxygen are as timely today as they were in the late 18th century.
The six characters in 1777 are Lavoisier (34 years old); Marie Anne Pierrette Paulze Lavoisier (1758–1836), his wife (19 years old); Priestley (44 years old); Mary Priestley, his wife (35 years old); Scheele (35 years old); and Sara Margaretha Pohl (Scheele’s housekeeper, whom he married three days before his death). The five fictional characters in 2001 are Bengt Hjalmarsson (played by the same actor as Lavoisier); Sune Kallstenius (played by the same actor as Scheele); Astrid Rosenqvist (played by the same actress as Mrs. Priestley); Ulf Svanholm (played by the same actor as Priestley); and Ulla Zorn, supposedly an amanuensis to the Chemistry Nobel Prize Committee of the Royal Swedish Academy of Sciences but later revealed to be a graduate student in the history of science at Lund University who is writing a Ph.D. dissertation on “Women in the lives of some 18th-century chemists” (played by the same actress as Fru Pohl). The first four characters are professors and members of the committee, and Rosenqvist is its chairwoman.
In keeping with Djerassi’s feminist concerns (He calls himself the “Mother [not the Father] of the Pill”), the role of women in society in general and in science in particular is a key theme of the play, and the actresses are more important than the actors. When the Nobel Chemistry Committee members ask who should be consulted about the true discoverer of oxygen, the mysterious Ulla Zorn suggests, “Most men around that time had wives. Why not look for what they had to say?” (p 18). When the idea surfaces that they should search for “dirt,” Zorn, in a cogent double entendre, suggests that they look to the wives: “Aren’t they usually expected to clean up the dirt?” (p 32).
The action begins with the women. In Hoffmann’s words, “We are introduced to the men in the 18th century through the women” [15]. Mme. Lavoisier, who played an active role in her husband’s research and who emerges as the central character of the play [19], Mrs. Priestley, and Fru Pohl are sharing a sauna (a locale for which Djerassi seems to have a predilection, having used it in his third novel, Menachem’s Seed [20]), where they discuss their backgrounds, activities, and their relations with the men in their lives. A pivotal point in the story quickly emerges as Fru Pohl asks Mme. Lavoisier, “Did Herr Scheele not send three years ago a letter to Paris describing his experiment with Fire Air?”, to which Mme. Lavoisier replies, “I know of no correspondence between them” (p 8). In Intermezzo 1, which connects the previous Scene 1, with Scene 2, Mme. Lavoisier soliloquizes, “She knows of the letter, our Mme. Pohl. (Pause). I am afraid” (p 9).
This and another important letter figure prominently in the play and reflect on the claims of priority. The first, a letter in French dated September 30, 1774 from Scheele to Lavoisier describing his discovery of oxygen is the earliest known description of the detailed method for preparing the gas along with information on its physical and chemical properties (pp 59–63) [21]. The letter is reproduced on slides in the performed play (Scene 7) and on pages 60 and 62 of the book. The Nixonian Watergate question immediately arises: What did Lavoisier know, and when did he know it? However, it is not known if Lavoisier ever read it.
The second letter, a fictional one that was “apparently never sent,” which Ulla Zorn found, is supposedly contained in Mme. Lavoisier’s travel chest disguised as a book titled “Histoire des Théatre” (The chest actually exists in the Cornell University Lavoisier Archives, and it is shown in photographs on pp 95–98). According to Zorn, “Apparently she intercepted Scheele’s famous letter…remember she handled much of Lavoisier’s correspondence” (p 99). Mme. Lavoisier appears (Characters from one time frame sometimes appear in the other) and reads from her letter,
I ask you now to forgive me. I could not show Apothecary Scheele’s letter to you, my dear husband. It would have taken the wind out of your sails, you, who were so close…And I told you why I felt incapable of destroying it. Our priority rested on my hiding it (p 99).
Zorn states, “Note! She didn’t say ‘your priority’…but ‘our’” (p 100), thus emphasizing Mme. Lavoisier’s active participation in her husband’s work. Later Astrid Rosenqvist refers to the limitations that women then faced: “but we all know what role women played in chemistry at that time. Madame Lavoisier got about as close as was realistic” (p 114).
The problem of balancing scientific work with family faced by women scientists is perennial and timeless. In 1777, when Mme. Lavoisier enumerates the work that she did for her husband, Mrs. Priestley compassionately asks her, “Is that why you have no children?” (p 6), while in 2001, the following exchange occurs:
Ulla Zorn: I just wanted to know what price you’re willing to pay to be a successful scientist…and as a woman.
Astrid Rosenqvist: I have no children. Many would consider that a heavy price.
Ulla Zorn: Like Mme. Lavoisier? (Pause). Is the committee your child? (p 35).
Oxygen even contains a short masque or “play within a play” à la Hamlet in verse for Gustav III and his court (pp 42–45), in which “Oxygen” (played by Mme. Lavoisier) overcomes “Phlogiston” (played by Lavoisier), based on such a play, now lost, that the Lavoisiers performed for their friends and patrons.
In Djerassi’s words, “There are seven alternatives for our retro-Nobel: it could be awarded to the three people individually, three pairs, or all three together” [15], but we do not learn the committee’s final decision. In our opinion, the ambiguity in the play’s ending is deliberate and reflects Djerassi’s discontent with cut-and-dried solutions His words, spoken at the end of his first play, An Immaculate Misconception, are applicable to the issues of discovery, priority, and the manner in which scientists conduct themselves that are the focus of Oxygen:
These are all gray issues. There are no black and white answers. And there is the question I really would like to ask the spectators to ask themselves. And the answers, in my opinion, cannot be provided by scientists, cannot even be provided by governments. I think the answer can be provided by individuals, based on reasonable information. And one of the attempts—perhaps the main attempt—of my play, aside from amusing you, is to actually inform you so that you are better informed to make complicated decisions about enormously complicated and ethically charged problems [22].
We are extremely pleased to note that the progress that Djerassi and Hoffmann have made individually in their creative writing has carried over to their first collaborative effort. The plot, based in part on actual history of science, is complex and absorbing, and the characters, although flawed, are interesting human beings and ring true to their historical counterparts. According to Hoffmann,
People want to understand what has driven scientists, how that precious knowledge has been gained, and whether one has had to sacrifice some aspect of one’s humanity to gain it. That’s what we address in our play [15].
We think that Djerassi and Hoffmann have admirably succeeded in attaining their goal.
The main themes that we have described above are skillfully and consistently woven throughout the dialogue, which, although often didactic, is never “stuffy.” In their play, touted as an examination of “priority, politics, passion,” Djerassi and Hoffmann have helped bridge the gap between “the two cultures” by giving a general audience an insightful and accurate view of how scientists act and how science advances—a picture that is often at odds with the public’s stereotyped view of scientists as cold, unemotional, ultra-rational, unselfish, “do-gooders,” who are driven solely by disinterested curiosity. As Mme. Lavoisier says in 1777, “the product of science is knowledge…but the product of scientists is reputation” (p 5), while Astrid Rosenqvist says in 2001, “Science is done by humans…humans are competitive…human scientists are even more competitive…and they want to be rewarded for being first” (p 109).
We give Oxygen, an enjoyable, engrossing, and above all, provocative and thought-provoking play, an enthusiastictwo thumbs up [23].
References and Notes
1. Kauffman, G. B.; Kauffman, L. M. The Steroid King. The World & I 1992, 7 (7) (July), 312–319.
2. (a) Djerassi, C. An Immaculate Misconception: Sex in an Age of Mechanical Reproduction; Imperial College Press: London, 2000; (b) Kauffman, G. B.; Kauffman, L. M. Chemical Educator 2002, 7, 245–248; DOI 10.1007/s00897020589a.
3. Lustig, H.; Shepherd-Bard, K. Science as Theater. Am. Scientist 2002, 90, 550–555.
4. For an annotated list of “science plays” with capsule summaries and bibliographical information, along with links to Internet resources for further exploration of “Science as Theater,” access http://www.americanscientist.org/articles/02articles/lustig.html.
5. Kauffman, G. B. Roald Hoffmann. In Biographical Encyclopedia of Scientists; Olson, R.; Smith, R., Eds.; Marshall Cavendish Corporation: New York, 1998, Vol. 3, pp 635–637.
6. Hoffmann, R. The World of Chemistry; Intellimation: Santa Barbara, CA, 1989; Kauffman, G. B. J. Chem. Educ. 1990, 67, A54–A55.
7. Hoffmann, R. The Metamict State; University of Central Florida Press: Orlando, FL, 1987; Kauffman, G. B. J. Chem. Educ. 1989, 66, A47;
8. Hoffmann, R. Gaps and Verges; University of Central Florida Press: Orlando, FL, 1990; Kauffman, G. B.; Kauffman, L. M. Angew. Chem., Int. Ed. Engl. 1990, 20, 1488–1489.
9. Hoffmann, R. Memory Effects; Calhoun Press (Columbia College): New York, 1999.
10. Hoffmann, R. Soliton; Truman State University Press: Kirksville, MO, 2002.
11. Hoffmann, R. Catalísta; Huerga y Fierro: Madrid, 2003 (a bilingual book of Hoffmann's poems in Spanish translation).
12. Hoffmann, R.; Torrence, V. Chemistry Imagined: Reflections on Science; Smithsonian Institution Press: Washington, DC, 1993; Kauffman, G. B.; Kauffman, L. M. J. Chem. Educ. 1995, 72, A18.
13. Hoffmann, R. The Same and Not the Same; Columbia University Press: New York, 1995; Kauffman, G. B.; Kauffman, L. M. J. Chem. Educ. 1996, 73, A47.
14. Hoffmann, R.; Schmidt, S. L. Old Wine, New Flasks: Reflections on Science and Jewish Tradition; W. H. Freeman & Co.: New York, 1997; Kauffman, G. B.; Kauffman, L. M. J. Chem. Educ. 1998, 75, 1097–1098.
15. Discovering Oxygen The Dramatist 2001 (Nov/Dec), 4 (2), 16–28 (http://www.djerassi.com/oxygen12/index.html) [an edited transcript of a conversation conducted by Dorian Devins, host of station WMFU’s “The Green Room,” with Djerassi and Hoffmann].
16. The American Chemical Society honored Priestley by naming its highest award the Priestley Medal, which both Djerassi and Hoffmann have won.
17. The event is so important that the Priestley Centennial meeting, held on July 31, 1874 at Northumberland, Pennsylvania, where Priestley had died in 1804 after fleeing from England, resulted in the founding of the American Chemical Society less than two years later.
18. Scheele, C. W. Chemische Abhandlung von der Luft und dem Feuer. Nebst einem Vorbericht von Torbern Bergman; Uppsala and Leipzig, 1777; also in Sämmtliche physische und Chemische Werke, Hermbstädt, S., transl.; Martin Sändig: Wiesbaden, 1971; Vol. 1, pp 13–200; Chemical Observations and Experiments on Air and Fire by Charles-Wilhelm Scheele…with a Prefatory Introduction by Torbern Bergman; translated from the German by J. R. Forster; J. Johnson: London, 1780; for excerpts translated into English see The Discovery of Oxygen, Part 2: Experiments by Carl Wilhelm Scheele (1777); Alembic Club Reprint No. 8; E. & S. Livingstone: Edinburgh, Scotland; 1952.
19. Mme. Lavoisier is the only one of the three women who has earned a place in The Biographical Dictionary of Women in Science: Pioneering Lives from Ancient Times to the Mid-20th Century; Ogilvie, M.; Harvey, J., Eds.; Routledge: New York/London, 2000; Vol. 2, pp 752–753.
20. The letter, discovered among Lavoisier’s papers by his biographer, Édouard Grimaux, who published it (Une lettre inédite de Scheele à Lavoisier. Revue générale des sciences pures et appliquées January 1890, 1, 1), is preserved in the archives of the French Académie des Sciences. See Boklund, U. Carl Wilhelm Scheele. In Dictionary of Scientific Biography; Gillispie, C. C., Ed.; Charles Scribner’s Sons: New York, 1975; Vol. 12, pp 143–150.
21. Djerassi, C. Menachem’s Seed; the University of Georgia Press: Athens, GA, 1998; Kauffman, G. B.; Kauffman, L. M. J. Chem. Educ. 1998, 75, 1096–1097.
22. An Immaculate Misconception: Sex in an Age of Mechanical Reproduction, a VHS videotape of an April 7, 2000 performance at the Alcazar Theatre, San Francisco, directed by Libby Pratt and with commentary by Djerassi (ISBN 1-892045-08-7; 74 min., $27.00, NTSC; $30.00, PAL) is available from Globalstage Productions, Inc., 1210 Union Street, San Francisco, CA 94109 (telephone: 1-888-324-5623; email: info@globalstage.net; Web site: http://www.globalstage .net/).
23. Djerassi’s third play, Ego, described as a “a dark comedy about a singular obsession,” directed by Frances McCain and presented by PlayBrokers with the Playwright's Foundation, premiered as a reading on February 10, 2003 at the ODC Theatre in San Francisco, CA 94110 followed by a discussion with the author, the actors, and the director. Djerassi describes this effort as “my first experiment in ‘nonscientific’ play writing” (email to G. B. Kauffman, January 26, 2003).
George B. Kauffman and Laurie M. Kauffman
California State University, Fresno, georgek@csufresno.edu
S1430-4171(03)02680-3, 10.1333/s00897030680a
Practical Laboratory Skills Training Guides. Elizabeth Prichard, co-ordinating author. The Royal Society of Chemistry: Cambridge, UK, 2003. $89.95, £70. ISBN 0-8540-4458-2.

This series consists of five booklets, each dealing with a specific subject relevant to modern analytical work. According to the back of the booklets, the aim of the series is “to make achieving best practice easy,” and they are meant to enable both experienced and inexperienced scientists to get any experiment right. This seems to be a huge claim to live up to. As a result, it seems best to examine each of the five booklets individually to discover if they actually do.
High Performance Liquid Chromatography
This 35-page booklet is split into 3 sections, which cover the theory of HPLC, the components of HPLC systems, and contain a problem-solving guide. The theory section is comprehensive and touches on the mathematics involved in the separation of compounds. This would show to the novice why some factors, such as length, affect retention times and, therefore, the effectiveness of the separation of compounds in mixtures; however, there are no practical procedures to illustrate these points.
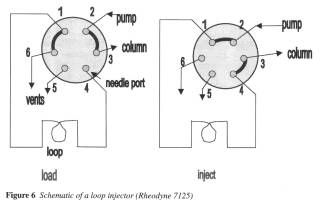
The bulk of this booklet deals with the components of the HPLC system, namely subsections on the mobile phase, the pump, the injector, the column, and the detector, with most of the text focused on the type of column to use.
The subsection on the mobile phase deals mainly with the preparation and handling of the solvents to be used and it includes a useful table of common solvents used in HPLC including the solvents’ UV cut-off point and polarity index. There is a list of main points (or “practical tips” as it is called in the booklet) that would be invaluable to an inexperienced scientist when they begin to prepare the mobile phases themselves, but would soon become second nature as the person gains experience and, therefore, would make the section redundant quickly. It would have been particularly helpful to have some examples of which solvent(s) to use for particular compounds. There is one practical procedure included that illustrates the effect of changing the percentage composition, but there is nothing practical to demonstrate the effect of actually changing the solvent, for example, from acetonitrile to methanol.
The subsections on the pump and the injector are concise and only really consist of tips on how to look after these pieces of equipment. Again, this would be useful to an inexperienced user but would quickly become automatic and the text would, therefore, quickly lose its usefulness.
The detector subsection deals mainly with UV detection with a mention of refractive index (RI) and a table of other detector types tagged on at the end. This is useful information to have for a better understanding of the subject of HPLC, but it is not particularly relevant in a training guide. Maybe a practical experiment should have been included to show that components could be missed if a UV detector is used instead of a RI detector.
The subsection on the types of columns goes into great detail about the different types of stationary phase available and includes parts on different pore sizes and particle sizes but does not go into any real detail about how these factors affect the separation. This subsection, like all the others, has a section dedicated to practical tips on looking after the column. Again, the usefulness of this section would probably be short-lived as most points made are fairly obvious.
The most important part of this would be expected to be the practical training section; however, this doesn’t seen to be what the authors were thinking, because the section that actually deals with the practical side of HPLC is given a single page and is buried in between system parameters and calibration. This really should have been given a more obvious title, for example, “HPLC Experiments,” and because this is a training guide, it would be expected that the experimental section would be a set of procedures instead of a list of ideas.
The best part of the booklet is the section that deals with overcoming common problems seen in HPLC spectra. Each problem is clearly illustrated and a list of checkpoints is given that would no doubt save the everyday analyst a lot of valuable time. This section undoubtedly makes the whole booklet useful to any scientist who is performing HPLC whether inexperienced or a regular HPLC user.
Measurement of Volume
This 22-page booklet is split roughly into 3 parts: an introduction to the concept of accurate measurement of volume and the jargon involved, a section on the different types of equipment currently available for measuring volumes accurately, and a section on how to use the pieces of equipment with the majority of the book dealing with the different types equipment for measuring volume.
The introduction, especially the section on the jargon of the subject, is written in a very clear manner making it extremely easy-to-read. This section would be very useful to someone new to the subject as well as someone who is refreshing their memory on the terminology used.
The next section of the booklet deals with the various types of equipment available, how the equipment is calibrated, and finally how to the check the accuracy of that equipment. This section is helpful in trying to decide what piece of equipment to use in any given situation; however, this would probably only be useful to an inexperienced person because it is all pretty obvious stuff. The information on checking the accuracy of the equipment is invaluable and probably makes this booklet worth having.
The final section deals with how the piece of equipment is actually used to deliver the required volume. This section is written in a bullet-point manner, making it extremely easy to follow. This section should be useful to anyone, whether it be someone new to that piece of equipment or someone brushing up on their techniques.
Measurement of pH
This 18-page booklet is very similar in setup to the measurement of volume booklet, that is, containing the same 3 sections but dealing with pH instead of volume.
The introduction deals mainly with explaining what pH means and the mathematics used by a pH meter/electrode to calculate the pH of a solution. This is clearly written and as a result is easy to follow, although its usefulness in a practical training guide is questionable as all it does is explain how a meter actually works. There is also information on buffer solutions used to calibrate the instrument. Again, this is all clearly explained, but mostly irrelevant in a training guide. It would have been more useful to have also included a section on how to make various pH buffers from chemicals around the laboratory.
The next part of the booklet deals with the different types of meters and electrodes available and focuses heavily upon pH electrodes. The most important section here is the part that deals with looking after the equipment. The section is clearly written so that anyone could follow the ideas expressed within and would undoubtedly be useful to all levels of experience.
The final part of the booklet explains what criteria to use when choosing the type of pH-measuring equipment to use and how to actually use that piece of equipment. As with the rest of the booklet, this is written in a straightforward manner and all instructions are very easy to follow.
Measure of Mass
This 16-page booklet has a similar layout to the other 2 measurement booklets; however, the question that has to be asked is, are 16 pages really needed to discuss how to use a balance to get an accurate mass? Because all three measurement booklets are written by the same author, the same straightforward language is used in this booklet and, therefore, the booklet is well written; however, it does not really say anything. The sections that deal with the terminology used in mass measurements and where to actually place your balances are all nicely defined and the section on choosing the correct type of balance are of the utmost importance, but most of the rest of the information within seems to be irrelevant in a training manual.
Gas Chromatography
This 36-page booklet, like the rest of the series, is split roughly into 3 sections: the theory of GC, the components of GC systems, and a problem-solving guide. The theory section is very comprehensive and deals with the mathematics involved in the separation of compounds. There are clear diagrams that illustrate the origin of all the factors involved in the calculations. Although worthwhile knowing, this would seem to be irrelevant in a training guide, unless the ideas given were then used to prove something.
The main bulk of this booklet deals with the components of the GC system, namely, subsections on the carrier gas, the injector, the column, the oven and the detector.
The subsection on the carrier gas deals mainly with choice of gas and why it should be as high a purity as possible. Although this is of utmost importance, it does seem to be irrelevant to a training manual, because the person in charge of the maintenance of the system will have undoubtedly purchased the correct gas and have systems in place to ensure that impurities such as oxygen are removed.
The subsections on the types of injectors and sampling methods are clearly explained and contain some very important information, but again the question of relevancy must be raised. This is especially true when it comes to the type of injector used because the GC system would already be in place and the injector system would have been chosen by the purchaser of the machine. It does seem very strange that various types of sampling methods are listed yet in this so-called practical training guide there are no experiments given to show the difference between them. It is also strange that no methods are given for the creation of volatile derivatives when a list of derivatizing agents is given.
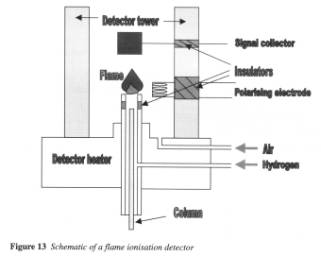
The detector subsection has nicely annotated diagrams that tie in well with the text about each type of detector available. This is useful information to have for a better understanding of the subject of GC, but it is not that relevant to a practical training guide. The subsection on the programming of the oven is excellent and is backed up with a section of practical experiments. This is extremely useful in illustrating the points the authors are trying to make.
Unlike the HPLC booklet, this one has very little detail on the types of column, that is stationary phases that are available to use; basically, it is just a list. There is an excellent list of bullet-pointed questions that should be asked when choosing a column that would be useful to anyone using GC. Maybe if there had been an experiment that used this information to demonstrate what occurs if the wrong column is chosen, the list would have had an even greater value.
Like the HPLC booklet, the experimental procedures included in this booklet are limited. The experiments given are useful to a novice who is trying to understand how GC works, but as mentioned elsewhere in this review, it is a shame that no derivatization experiments are present.
Like the HPLC booklet, the best part of this booklet is the section that deals with overcoming common problems seen in GC spectra. Again, this section would no doubt save the everyday analyst a lot of valuable time and, therefore, makes the whole booklet useful to any scientist, whether inexperienced or a regular GC user.
Overall review
As can be seen in the individual reviews, each of the five booklets are clearly written and do contain some sections that make purchasing them worthwhile; however, it seems as if the authors could not make up their minds about what they are trying to produce. According to the back of the booklets, theory is kept to a minimum, yet in some cases there is far too much theory, for example, about the different types of detectors available, and yet in other cases not enough, which could easily confuse a novice.
The major problem with the series is that the booklets claim to be practical training guides. If this were the case, then complete novices should be able to read them and then be able to actually perform the experiment themselves. Without other sources of information, however, perhaps an expert in the field, this would not be the case. There just is not enough practical training within these booklets.
This series seems to be a wasted opportunity. With a little more theory included and the price lowered, these would have been an excellent series of textbooks for an analytical course for undergraduates or, if the practical procedures had been expanded, this would have been an excellent training guide. Unfortunately, it fails on both counts.
Malcolm Stewart
Oxford University, malcolm.stewart@chem.ox.ac.uk
S1430-4171(03)02681-2, 10.1333/s00897030681a