 The
Chemical Educator, Vol. 8, No.3,
Media Reviews, © 2003 The Chemical Educator
The
Chemical Educator, Vol. 8, No.3,
Media Reviews, © 2003 The Chemical Educator
Media Review
 The
Chemical Educator, Vol. 8, No.3,
Media Reviews, © 2003 The Chemical Educator
The
Chemical Educator, Vol. 8, No.3,
Media Reviews, © 2003 The Chemical Educator
Media Review
Broadening Electrochemical Horizons. ByAlan M. Bond.Oxford University Press: New York/London, 2002. paperback, £45.00, $80.00 U.S. ISBN: 0198504772.

In the preface, Alan Bond describes his motive for writing this epic, namely, his delivery of the Hinshelwood Lectures. This lecture series, named after Cyril Norman Hinshelwood (Nobel Laureate in Chemistry, 1956), which is held annually at the University of Oxford, gives the invited speaker an opportunity to present his or her work in the area of physical chemistry to a general chemistry audience. The author was the Hinshelwood lecturer in 1998, presenting a series of lectures entitled “Broadening Electrochemical Horizons,” which were aimed at illustrating the principles and applications of electrochemical methodology.
The first chapter entitled “The Fundamentals of Electrochemistry,” provides a comprehensive table on the development of electrochemistry, charting the days from Galvani, Davy, Faraday, and onwards to Heyrovský and later. The second table in the section has an amusing adaptation of dynamic electrochemistry, a“good versus bad” of experimental electrochemistry. This chapter is well written, illustrating the basic theory needed for understanding the later chapters, and it finishes with basic and important principles of fuel cells, photovoltaic cells, and the lead-acid battery.
In chapter 2, the principles of voltammetry, electrolysis, and other techniques necessary for the understanding of electrode processes are discussed. The start of the chapter appears in similar form elsewhere, but the latter part of this chapter is truly excellent, perhaps one of the highlights of this text. It directs the reader to some superb references while encompassing a wide range of complementary techniques, such as spectroelectrochemistry. In addition, there are some excellent schematics of experimental cells. There is also an elegant subsection on the electrochemical quartz-crystal microbalance with basic theory and applications, ending with a discussion of the use of scanning probe microscopy to observe what is happening on electrochemical surfaces during voltammetric interrogation. The only criticism is that, due to its sheer volume, this chapter might better have been broken into two parts.
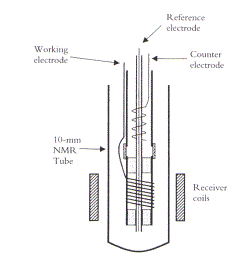
Figure 1. Just one of the many elegant schematics, this example is of an NMR spectroelectrochemical cell described by Bond in chapter 2.
Progressing onto chapter 3, “The basics of voltammetry for solution-soluble species,” the author uses polyoxometalate compounds as a model for explaining adverse phenomena. These are used to demonstrate how voltammetry, simulation, and spectrochemical techniques can be used to probe complicated electrochemical species. This chapter is rather lengthy, more protracted than it needs be, and the reader’s mind wanders. The subsequent section picks up pace slightly and discusses anodic and adsorptive stripping voltammetry with mercury films. This segment has a little theory thrown in for good measure with a rather elaborate discussion of the mechanisms associated with adsorptive stripping voltammetry of the classical nickel dimethylglyoxime complex at a mercury electrode. This chapter concludes with a comprehensive discussion of how voltammetric techniques can be applied in the real world in the form of glucose biosensors.
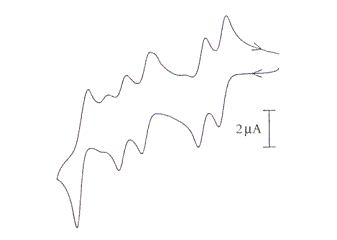
Figure 2. Cyclic voltammogram for the reduction of 2 mM [S2Mo18O62]4- which Bond uses to demonstrate the basics of voltammetry in chapter 3.
Chapter 5 of this book, probably the most appealing of the later chapters, gives the reader a taste of solids on electrode surfaces. The chapter deals adequately with solid-state transformation by application of a variety of techniques, with mechanistic aspects of transport processes discussed thoroughly. Chapter 6 draws the book to a close with a discussion on metalloprotein voltammetry. This is illustrated by blocked electrodes, with a discussion on the self-blocking mechanism of cytochrome c, and it finishes with modified electrodes illustrated in the detection of various cytochromes.
To summarize, this book is mostly well written and the early chapters are excellent, providing a first-rate reference for all aspects of experimental electrochemistry. Although the later chapters may be too specialized for some, the fundamental chapters are a definite must for masters or Ph.D. students of the subject.
Craig Banks
Oxford University, Physical and Theoretical Chemistry Laboratory, craig.banks@chem.ox.ac.uk
S1430-4171(03)03693-X, 10.1333/s00897030693a
Chemistry for Dummies. By John T. Moore. Wiley: Indianapolis, IN, 2003. paperback. $19.99. ISBN: 0-7645-5430-1.

For readers who are not familiar with the Dummies series of books from Wiley Publishing, they are purported to be “A Reference for the Rest of Us.” This popular series of books began as computer software help manuals (DOS for Dummies, Excel for Dummies, etc.) and later was extended to unlikely things like Guitar for…, Diabetes for…, and even Sex for…. The advertised features of a “Dummies” book are: (1) explanations in plain English, (2) “get in, get out” information, (3) icons and other navigational aids, (4) tear-out cheat sheet, (5) top ten lists, and (6) a dash of humor and fun.
This brief introduction to the “Dummies” series is necessary in order that I might explain in this review whether or not I think you want a book to do this for you in the area of chemistry and if this book has indeed fulfilled its promise.
In the software manual world, where these simplified-explanation books originated, there are indeed simple ways to say the arcane ramblings of computer nerds. Phrases like “make this the default,” “format as FAT32,” or “create a macro,” can usually be rephrased into simple and familiar words or phrases; however in chemistry such translation of the chemist’s vocabulary into simple and familiar words is far from easy. Introductory chemistry courses are dedicated to the task of building a careful vocabulary of words early in the course so that the learner might comprehend the meaning of later, more arcane, words that arise as the course gets deeper into the subject. A chemistry textbook is not a “get in, get out” kind of book; a
dictionary is. As for a “tear-out cheat sheet,” in this dummies book it is no more than a small periodic table with a few formulas written on the reverse side.
Now it is only fair that I assume from this point that the reader does want a simple book from which to become familiar with some of the terminology of chemistry, rather like a very easy-to-read high school chemistry text. Is this that book? Perhaps it is. This book has some humor in it and a not-so-serious approach that shows how hard the author tried to do what the dummies series editor asked of him. Part IV: “Chemistry in Everyday Life: Benefits and Problems,” is a section that a nonchemist certainly might read with profit and enjoyment.
Unfortunately the book is rather like the lectures of a pleasant professor who has been assigned to cover all of chemistry in three class meetings and with no laboratory instruction. It’s a nice try, and he gets the words all in, but in the end these chemical words don’t mean much more to the class than they did earlier. Explanations are necessarily very superficial. There is a lot of “here’s how you do this stuff” when a new topic is introduced, but not much of why you would want to do this.
Because there are no problems in the book, there are, consequently, few errors; however, the electrochemistry chapter starts with arbitrary and unexplained rules, (there are rules for what oxidation is, but there are no rules for reduction) and no explanation as to why one might need these rules. When, finally, the Daniell cell is encountered, the conceptual errors begin to creep in. The most egregious of these is the connecting of the electrodes of the cell with a wire. Most people take the word “wire” to mean a conductor of almost zero resistance. Anyone who has actually worked with electrochemical cells knows not to do this. This shorts out the cell, whereupon it liberates all its potential energy as useless heat immediately. The voltage output of the cell is immediately zero, and all discussion of its value as an energy source becomes irrelevant. The cell terminals should instead be connected with a resistor, or a high resistance voltmeter, for the discussion of what is happening in the electrochemical cell to make any sense. Additionally, it is stated that “You can get just a little more voltage if you make the solutions that the electrodes are in very concentrated.” This is not true, nor is it true that the distinction between cells and batteries is “technical stuff.”
Errors aside, the book will do no harm to its readers. They may enjoy it. If you are a nonscientist invited out to dinner with a chemist and you want to drop some chemical words into the conversation, then by all means buy and read this book. It costs much less than the dinner will.
Roy W. Clark
Middle Tennessee State University, Murfreesboro, royclark@bellsouth.net
S1430-4171(03)03694-9, 10.1333/s00897030694a
Vacuum Technology Calculations in Chemistry. By D. J. Hucknall and A. Morris. Royal Society of Chemistry: Cambridge, England, 2003. Softcover, x + 234 pp. £39.50. ISBN 0 85404-651-8.

Vacuum technology is used widely in experimental science and technology, yet most who are involved with it undergo little formal training in the subject. Manufacturer’s leaflets, tips picked up from working with existing vacuum systems, hours spent trying to figure out some system malfunction—these are the typical sources of knowledge that many of us draw on in working with this technology. An alternative approach is to resort to textbooks on the subject, and this new book, by Hucknall and Morris, represents a further addition to the modern literature in this field.
The material presented starts with the kinetic theory of gases and an introduction to transport properties and gas dynamics; it then moves on to the calculation of gas flows through vacuum components, considering viscous, molecular, and intermediate flow regimes. The next chapter presents a discussion of some widely used pumping systems, specifically various roughing pumps and high/ultra high vacuum pumping including diffusion, turbomolecular, and cryopumps, along with getter and sputter ion pumps. A discussion of the unavoidable gas sources in vacuum systems and how this influences the lowest gas pressures obtainable is presented. Leaks, permeation, and outgassing are all discussed. Pressure measuring devices are covered, with a brief consideration of the range of direct measurement devices; a description of some indirect devices, such as Pirani and ionisation gauges; and measurement of partial pressures using quadrupole mass spectrometers. Some of the applications of vacuum in various chemical technologies are looked at in detail. The case studies include distillation and evaporation, the attainment of ultra-high vacuum, and the use of differential pumping in such areas as molecular beams. A summary of the book content then completes the edition.
Who will find this book useful? The range of vacuum technology covered is quite wide, but the level of detail is not high. As a reference book, it thus provides a useful overview to the uninitiated in the area. Unfortunately, anyone wanting to use it to complete a design task or formulate some operating protocols would probably quickly have to resort to much more detailed information.
However, the real distinguishing feature and strength of the book, which is reflected in the title, is the numerical calculations, based around such topics as gas flows and pumping characteristics, which occupy a large fraction of the text. These are used to exemplify the relevant mathematical formulas that are used in this area of technology and include many very practical problems, which everyday confront practitioners in the field. I have not seen any other concise textbook that is so useful in this respect. For this reason, I will keep this book in a reasonably accessible place on my bookshelf.
John Foord
Oxford University, Physical & Theoretical Chemistry Laboratory, John.Foord@chem.ox.ac.uk
S1430-4171(03)03695-8, 10.1333/s00897030695a
The Road to Stockholm: Nobel Prizes, Science, and Scientists. By István Hargittai. Oxford University Press: London/New York, 2002. Illustrations. xvii + 342 pp. 16.1 ´ 24.0 cm. Hardbound, $29.95, £19.99. ISBN 0-19-850912-X.

Beginning in 2001 with the centenary of the Nobel Prizes, “the only science award, worldwide, that is appreciated by the general public, not just the scientists” (p 1), a large number of books dealing with the prizes as a whole and with some of their specific aspects have poured forth from presses around the world. In our opinion, of all these that we have read to date, The Road to Stockholm is the best general book on the science prizes [1]. István Hargittai modestly admits that he is not a historian or sociologist and calls his book “a personal account rather than a historical study.” However, it is a gold mine of information and insights for the scientist, historian of science, and sociologist of science as well as the public at large. It might well be titled Everything You Always Wanted to Know about the Nobel Prizes But Were Afraid to Ask.
Since his first interview with a Nobel laureate—that of Nikolai Nikolaievich Semenov (Chemistry, 1956) —conducted in Budapest in September, 1965, Hargittai, Professor of Chemistry at the Budapest University of Technology and Economics, Research Professor of the Hungarian Academy of Sciences at Eötvös University, and an interviewer par excellence, has immersed himself firsthand in all manner of Nobeliana. For his influential but unfortunately short-lived magazine, The Chemical Intelligencer (January, 1995–July, 2000) he interviewed 120 Nobelists, sometimes with his wife and frequent collaborator Magdi, to whom the present book is dedicated. Many of these interviews appeared or will appear in his projected four-volume series of books, Candid Science [2, 3]. For The Road to Stockholm alone he acknowledges the assistance of 76 laureates, four of whom were interviewed by Magdi. Throughout the book every laureate is consistently identified by year and field of his or her award and, on the first occurrence of the name in the text, with dates of birth and death. Hargittai also includes information from interviews with 42 other scientists.
Because his book contains so many personal reminiscences and recollections, Hargittai warns us that different persons may view or remember the same events in different ways. As James D. Watson wrote in his bestseller, The Double Helix,
I am aware that the other participants in this story [what has become known as “the Race for the Double Helix”] would tell parts of it in other ways, sometimes because their memory of what happened differs from mine and, perhaps in even more cases, because no two people ever see the same events in exactly the same light [4].
Watson, who shared the 1962 Nobel Prize in Physiology or Medicine with Francis H. C. Crick and Maurice H. F. Wilkins for the 1953 discovery of the double-helical structure of DNA, the 50th anniversary of which we celebrate this year, contributed the foreword to Hargittai’s book.
Although intended for the general reader, this extensively cross-referenced book is nevertheless meticulously documented with 46 pages of references to primary and secondary sources (Hargittai consulted archives in six countries), including websites and sources as late as 2002. It contains 59 plates; an annotated list of further reading; a list of all Nobel laureates in physics, chemistry, and physiology or medicine (1901–-2001) with the official citation for their awards; and a name index (10 double-column pages) but not a subject index.
The book evolved from a lecture, “How to Win a Nobel Prize” (not his choice of title) that Hargittai presented in Cambridge, England, sponsored jointly by the Laboratory of Molecular Biology, the home of so many Nobel laureates, and Peterhouse, the oldest college (founded in 1284) of Cambridge University. He also delivered an invited lecture on the subject of the book to the Royal Swedish Academy of Sciences on December 12, 2001, two days after the award ceremonies marking the centennial of the Nobel Prizes.
Hargittai has used much of the specific material from the interviews in the volumes of Candid Science to arrive at generalizations about the prizes and prizewinners, and the book is literally peppered with hundreds of memorable quotations from laureates and others. His knowledge of details is fantastic; we have written a number of articles about Nobel laureates, but even in these cases we found many facts, stories, and anecdotes of which we were previously unaware.
Chapter 1, “The Nobel Prize and Sweden” (28 pp), deals with Alfred Nobel’s will of November 27, 1895 that established the prizes; the “Nobelitis” (the “disease…that afflicts the person who is close, or thinks he is close, to getting the prize….making him miserable”) and “Nobelmania” (the disease that afflicts committee members who become “too obsessive about giving out the prize”); the monetary values of the prizes through the years; a day-by-day description of the prize festivities; the statutes governing the selection and awarding of the prizes; biases and politics in the awards; the nomination process; awards during war time; and related prizes such as the Crafoord, Wolf, and Lasker, and even the semiserious Ig Nobel prizes.
Chapter 2, “The Nobel Prize and national politics” (19 pp), considers the patterns in the countries represented in the awarding of the prizes, in particular, countries that have been underrepresented such as Japan and other non-western nations. The well-known phenomenon of the overrepresentation of Jews (only 14,000,000 or 0.2 percent of the world’s population) among laureates, despite the disadvantage of prevalent anti-Semitism, is addressed [5]. The special problems of laureates from Nazi Germany and the U.S.S.R. are considered.
Chapter 3, “Who wins Nobel prizes?” (35 pp, the longest chapter), deals with a variety of interesting topics. Alfred Nobel intended that the prizes be awarded for great discoveries rather than to great scientists or for a lifetime of achievements (Eugene P. Wigner (P 1963) and Gerhard Herzberg (C 1971) are notable exceptions) [6]. As a case in point Hargittai cites Kary B. Mullis’s (C 1963) discovery of the polymerase chain reaction (PCR) (A number of scientists think that Mullis, who has made antiscience statements, was not worthy of the prize). Although it is impossible to give a description of a typical Nobel laureate, in an unusual and novel section Hargittai compares several non-laureates and laureates to discern some characteristic traits of their personalities and careers—William Astbury and Linus Pauling (C 1954; PE 1962); Eugene Sverdlov and Walter Gilbert (C 1980); Yuval Ne’eman and Murray Gell-Mann (P 1969); Erwin Chargaff and James D. Watson (M 1962); and Charles Weissmann and Stanley Prusiner (M 1997). Hargittai also discusses laureates classified according to different types (drilling or digging), and he gives examples of laureates whom he calls “third persons”— those “positioned for the prize under fortunate circumstances and who is admissible because of the three-person rule makes it possible”—Robert Curl (C 1996), Robert Huber (C 1988), and John J. R. McLeod (M 1923)—as well as a missing ‘third person”— Robert B. Woodward (C 1965) in the 1973 chemistry award for sandwich compounds). Hargittai also discusses the characteristics of Nobel winners in terms of personality (humility is not one of them); scientific areas (basic science prevails over clinical discoveries in the physiology or medicine prizes); partners (mentors and students; spouses); gender (only ten women have won prizes in the sciences); longevity; and other qualities.
In Chapter 4, “Discoveries” (20 pp), Hargittai shows that prize-winning discoveries are often important, unambiguously attributable to one or a few persons, recognized at the time of the award, timely, or dogma- or paradigm-changing. In Chapter 5, “Overcoming Adversities” (14 pp), he contends that many of the laureates came from diverse backgrounds and struggled with various handicaps such as the Great Depression, illness, anti-Semitism, and the Holocaust. In Chapter 6, “What turned you on to Science” 12 pp, the shortest chapter), he discusses some of the factors that drew future Nobel laureates to science, including the inspiration provided by a book (especially Paul de Kruif’s Microbe Hunters (1926) and Sinclair Lewis’ Arrowsmith (1925), two of our own childhood favorites), a chemistry set (not only for future chemists) or other experimentation at home, a teacher (especially a high school teacher), the home environment, family members, a family friend, or other mentor.
Although the choice of adviser is crucial, Chapter 7, “Venue” (22 pp), deals with the environments conducive to an award-winning research career, in particular, the atmosphere, the presence of other professors and fellow students, the technological level of the institution, the visitors passing through, and especially, the research seminar. Among the most successful venues have been the Laboratory of Molecular Biology (LMB) and the Cavendish Laboratory, both at Cambridge University; Ernest Lawrence’s physics laboratory and G. N. Lewis’ College of Chemistry at the University of California, Berkeley; the Cold Spring Harbor symposia; the U.S. National Institutes of Health; the Rockefeller Institute (now Rockefeller University); the Wellcome Foundation laboratories in England; and the Pasteur and Max Planck institutes in France and Germany, respectively.
One of the most common features about the research careers of Nobel laureates is that many have worked under a former or future Nobel laureate or with someone closely associated with a laureate—a factor explored in a variety of cases in Chapter 8, “Mentor” (18 pp). Future Nobel laureates and other prominent scientists change the subjects and techniques of their research in a variety of ways as explored in Chapter 9, “Changing and combining fields” (15 pp). The change may be gradual or sudden, but the common characteristic is the willingness and ability to change. Among the careers discussed are those of Aaron Klug (C 1982), who worked in at least five areas; Jean-Marie Lehn (C 1987), who was an organic chemist before embarking on supramolecular chemistry; and the ultimate case of crossing disciplinary lines—Walter Gilbert (C 1980), whose career has ranged from theoretical physics to experimental biology and includes his role as a successful entrepreneur in biotechnology.
Once a discovery has been made, it must be disseminated so that others can use it and that eventually mankind can benefit from it. Chapter 10, “Making an impact” (17 pp), deals with the various ways of making it known. Coining a “catchy” name often helps to popularize a discovery, and Hargittai cites a number of such names and the persons who devised them such as buckminsterfullerene, prion, carbocation and magic acid, ATP, molecular biology, host-guest chemistry, macromolecule, and quark. He also makes the unexpected point that often future laureates faced difficulties in getting their (eventually) Nobel Prize-winning papers published in prestigious journals and cites a number of such cases. An important factor in creating an impact is the publicity of publications, and the number of citations received measures this publicity. However, although Nobel laureates had published frequently cited papers, Hargittai found no direct correlation between citation records and Nobel Prizes. Nonlaureate Oliver Lowry is the most cited scientist of all time for his 1951 article proposing a method for protein measurement, while the most cited British scientist at the end of the 20th century was Salvador Moncada for his work on nitric oxide as a signaling molecule in the cardiovascular system, but he was not included in the 1998 Physiology or Medicine Prize.
Receiving the prize definitely changes the life of the laureates. Chapter 11, “Is there life after the Nobel Prize?” (19 pp), deals with the various ways in which this occurs. The laureates have control over some of these and almost no control over others. Some laureates become increasingly involved in “outside” activities such as education, public service, political activism (Linus Pauling (C 1954; PE 1962) is the best known case), and areas beyond their field of expertise (for example, physicist William Shockley’s (P 1956) unscientific views on human intelligence and eugenics), while for others the prize’s notoriety helped them continue their scientific work long after retirement age.
In many ways Chapter 12, “Who did not win?” (27 pp), is the most interesting. While prizes are seldom awarded to undeserving scientists, many deserving scientists are overlooked so that the problem is one of omission much more than one of commission. Hargittai classifies nonwinners into those whose discovery was never honored by a prize and those who participated in making a discovery that was recognized but who were never awarded the prize, so-called “fourth persons” (at most three persons may share a prize). Among those in the first category who are discussed are Michael Tswett; Oswald Avery, Colin MacLeod, and Maclyn McCarty; G. E. Uhlenbeck and Samuel Goudsmit; Arnold Sommerfeld; G. N. Lewis, Walter Heitler, and Fritz London; Christopher K. Ingold; Jonas Salk and Albert Sabin; Edward Teller; Neil Bartlett; David Keilin; and Martin D. Kamen. Those discussed in the second category are Paul Ewald; Lise Meitner and Fritz Strassmann; Chen-Shiung Wu; Clyde Wiegand and Tom Ypsilantis; Rosalind E. Franklin; Freeman Dyson; Friedrich Hund; Kenneth Pitzer; Jocelyn Bell; Solomon Berson; Michael Fisher and Leo Kadanoff; Donald Huffman and Wolfgang Krätschmer; Salvador Moncada; and Oleh Hornykiewicz. We were familiar with a number of these nonwinners, but many of them were new to us.
In an “Epilogue” (4 pp) Hargittai summarizes his book and poses and answers several questions, for example: “Could science history be compiled on the basis of the Nobel Prizes? I think not.” “To what extent is the Nobel Prize forward-looking?” It honors “past achievements, whose validity has been reliably proved.” While Hargittai states that “there is no general recipe for getting the prize,” he concludes that “apart from the candidate’s talent and diligence the crucial step in a scientist’s career is the graduate school, including the choice of adviser” (p 249).
Hargittai prides himself on the care that he has taken in writing his 26 books, and the number of inevitable errors is remarkably small, considering the length and wide-ranging scope of this book: “Glen” for “Glenn” (Seaborg, p x); “1901” for “1900” (Gomberg’s discovery of free radicals, p 14); “sacrilege award” (words don’t make sense, p 49); “their” for “his or her” (p 51); “Lohman” for “Lohmann” (p 53); “maths” for “math” (p 120); “notabe” for “notable” (p 210); “regards” for “regard” (p 219); “Academie” for “Académie” (p 220); and “Ypsilanti” for “Ypsilantis (p 237).
According to Hargittai, “Our students, our children, the general public, all of us would benefit from knowing a little more about science and how it comes about because so much in our modern life depends on it” (p xii). We think that The Road to Stockholm goes a long way toward making this goal a reality.
References and Notes
1. Of course, other more specialized books deal in more detail than Hargittai with specific aspects of the prizes. For example, although throughout his book Hargittai discusses the problem of bias and national and international politics in the selection of Nobel laureates, this problem is understandably dealt with in more depth by Robert Marc Friedman in The Politics of Excellence: Behind the Nobel Prize in Science; Times Books, Henry Holt and Company: New York, 2001. For a review see Kauffman, G. B. Chem. Educator 2002, 7, 395–400; DOI 10.1007/s00897020643a.
2. Hargittai; I.; Hargittai, M., Ed. Candid Science: Conversations with Famous Chemists; Imperial College Press: London, England, 2000. For a review see Kauffman, G. B.; Kauffman, L. M. Chem. Educator 2002, 7, 184–186; DOI 10.1007/s00897020570a.
3. Hargittai; I.; Hargittai, M., Ed. Candid Science II: Conversations with Famous Biomedical Scientists;Imperial College Press: London, England, 2002; distributed by World Scientific Publishing Co.: Singapore; River Edge, NJ; London, England. For a review see Kauffman,G. B. Chem. Educator 2003, 8, 90–93; DOI 10.1333/s00897030663a.
4. Watson, J. D. The Double Helix: A Personal Account of the Discovery of the Structure of DNA; New American Library: New York, 1969, p ix.
5. Through 2000, 128 Nobel laureates—more than one-fifth of science laureates—were of Jewish parentage (21, chemistry; 37, physics; 39, physiology or medicine; 10, literature; 8, peace; 13, economics). For a list see Feldman, B. The Nobel Prize: A History of Genius, Controversy, and Prestige; Arcade Publishing Company: New York, 2000; pp 407–411.
6. In this review we abbreviate the laureates’ fields as C (chemistry), P (physics), M (physiology or medicine), and PE (peace).
George B. Kauffman and Laurie M. Kauffman
California State University, Fresno, georgek@csufresno.edu
S1430-4171(03)03696-7, 10.1333/s00897030696a