 The
Chemical Educator, Vol. 9, No.6,
Media Reviews, © 2004 The Chemical Educator
The
Chemical Educator, Vol. 9, No.6,
Media Reviews, © 2004 The Chemical Educator
Media Reviews
 The
Chemical Educator, Vol. 9, No.6,
Media Reviews, © 2004 The Chemical Educator
The
Chemical Educator, Vol. 9, No.6,
Media Reviews, © 2004 The Chemical Educator
Media Reviews
Uncle Tungsten: Memories of a Chemical Boyhood. By Oliver Sacks. Alfred A. Knopf: New York/Toronto, 2001. Illustrations. viii + 337 pp, hardbound, 14.5 ´ 21.9 cm. $25.00. ISBN 0-375-40448-1; Vintage USA: New York, 2004. paperback. $14.00. ISBN 0-375-70404-3.

Is there any chemical educator out there who hasn’t heard of Oliver Sacks (http://www.oliversacks.com), his lectures, his interviews, his books, and especially, his popular autobiography, Uncle Tungsten?
Sacks was born in London on July 9, 1933 into a family of physicians, metallurgists, chemists, and teachers, the members of whom figure prominently in Uncle Tungsten. He received his M.D. degree from Oxford University and since 1965 has been living in New York City, where he is a practicing neurologist, affiliated with the Albert Einstein College of Medicine in the Bronx, and an award-winning author. He received his latest honor, the American Crystallographic Association Science Writing Award, on July 21, 2004.
Sacks’ first book, Awakenings (Doubleday, 1974), describing his experiments treating catatonic patients with L-DOPA, inspired a play by Harold Pinter and was made into an Oscar-nominated 1990 movie of the same name, directed by Penny Marshall and starring Robert De Niro and Robin Williams. Most of his other books, A Leg to Stand On (Summit, 1984); Migraine: Understanding a Common Disorder (University of California Press, 1985); An Anthropologist on Mars: Seven Paradoxical Tales (Random House, 1995); The Island of the Colorblind and Cycad Island (Random House, 1997); and The Man Who Mistook His Wife for a Hat, and Other Clinical Tales (Touchstone, 1998), as well as Uncle Tungsten have been reprinted in paperback by Vintage Books, which, in 2004, issued Vintage Sacks, containing selections from five of his works.
Even before its publication as a hardcover volume, Uncle Tungsten appeared in The New Yorker, and Sacks and his book were profiled in the January 10, 2000 issue of Chemical & Engineering News [1], the cover of which featured a portrait of Sacks in his trade-mark periodic table T-shirt. Also, excerpts appeared in various publications, such as Chemical Heritage [2]. At its 226th National Meeting, held in New York City on September 7–11, 2003, the American Chemical Society celebrated the 125th anniversary of the Journal of the American Chemical Society and the 80th anniversary of Chemical & Engineering News with a special issue of C&EN devoted to the periodic table and the chemical elements [3]. Sacks signed copies of Uncle Tungsten and a special poster in honor of C&EN’s birthday on September 8. He also spoke about the book before the Division of Chemical Education [4]. Recently he read selections from Uncle Tungsten on C-SPAN-2’s “Book TV.”
The catalyst for the genesis of the book was 1981 Nobel chemistry laureate Roald Hoffmann. Roald sent Sacks a package containing a periodic table poster, a chemical catalog, and a bar of tungsten, which fell to the floor with a “resonant clonk:”
The clonk served as a sort of Proustian [the novelist Marcel, not the chemist Joseph Louis—GBK] mnemonic, and instantly brought Uncle Tungsten to mind….Other pictures rose immediately in my mind: his factories where the lightbulbs were made, his collection of old lightbulbs, and heavy metals, and minerals. And my own initiation by him, when I was ten, into the wonders of metallurgy and chemistry. I thought I might write a brief sketch of Uncle Tungsten, but the memories, now started, continued to emerge—memories not just of Uncle Tungsten but of all the events of my early life, of my boyhood, many forgotten for fifty years or more. What had started as a page of writing became a vast mining operation, a four-year excavation of two million words or more—from which, somehow, a book began to crystallize out (p 315).
After acknowledging a number of persons, Sacks concludes the volume with the following sentence:
But, above all, it has been Roald Hoffmann who has been infinitely stimulating and supportive, and who has done more than anyone else to show me the marvelous thing which chemistry is now—and it is to Roald, therefore, that I dedicate this book (p 320).
In his earlier books Sacks directed his attention outward, to patients and other persons. In his latest opus, a coming of age tale of a Jewish boy growing up in England during the years preceding and during World War II, he turns his supreme storytelling skill to concentrate on himself and his family and his preoccupation with the material world around him and the chemists and scientists who discovered the facts and principles of the sciences to which he was attracted. Being almost the same age as Sacks (I was born in 1930), and sharing his religion, adolescent absorption in the romance of science, the same books, a chemistry set [5–7] and home laboratory, and many more of Sack’s childhood experiences mutatis mutandis, I enjoyed the engrossing and nostalgic stroll down memory lane that his book afforded me. And many others of our era should find a similar pleasure in reading his scientific autobiography.
As most of us adolescent chemists, Sacks and I indulged in pyrotechnics, especially on holidays calling for fireworks—he on Guy Fawkes night (November fifth) and I on the Fourth of July. Even his unhappy stay at a boarding school to which he had been evacuated during his sixth to ninth years to escape the bombing of London was paralleled by my sojourn at a foster home during my ninth year.
I really envy Sacks, whose large family encouraged his interest in science (Aside from a chemistry set that my maternal uncle and aunt presented to me on my seventh birthday, I had to obtain chemicals and equipment on my own and often surreptitiously). In addition to his mother and father, Sacks’ two maternal uncles exerted a great influence on him. David Landau (the Uncle Tungsten of the title), who manufactured light bulbs with fine filaments of fine tungsten wire, introduced him to the mysteries of the metals that fascinated the young and budding scientist. Abraham Landau (Uncle Abe), a physicist, introduced him to cathode rays and allowed him to experiment with his powerful electromagnet, which enabled him to explore the relationship between magnetism and electricity (Sacks uses his experiences to discuss the contributions of Alessandro Volta, Michael Faraday, James Clerk Maxwell, and others).
Sacks utilizes his experiments to discuss the history of chemistry, of physics, of science, and of invention and the persons who made these contributions. In this personal context he recounts the stories of Robert Boyle, John Dalton, Madame Curie, Antoine-Laurent Lavoisier, Dmitrii Ivanovich Mendeleev [8], Michael Faraday, Ernest Rutherford, Joseph Priestley, and many others. Sacks and I both share Sir Humphry Davy, whose achievements we both attempted to reproduce in our home laboratories and to whom he devotes an entire chapter (pp 117–131) and more, as our scientific hero [9–12]. In his discussions of these and other scientists and their contributions, he presents in a historical context many of the fundamental concepts that are sine qua nons in any introductory chemistry, physics, or natural science course.
According to the late Harlow Shapley—an American astronomer, not a chemist—the periodic table
is probably the most compact and meaningful compilation of knowledge that man has yet devised. The periodic table does for matter what the geological age table does for cosmic time. Its history is the story of man’s great conquests in the microcosmos [13].
Sacks and I share a fascination with this central key to the mystery of the elements, their atomic weights, and properties. We each spent many hours during adolescence enthralled by a giant periodic table mural—he in South Kensington’s Science Museum and I in the Franklin Institute’s Hall of Chemistry in Philadelphia. We both began a collection of elements, although his was much more complete than mine. In the chapter titled “Mendeleev’s Garden” (Chapter 16), Sacks’ prose verges on the poetic or mystical, evocative of some of Primo Levi’s work:
The table was a sort of cosmic staircase or a Jacob’s ladder, going up to, coming down from, a Pythagorean heaven….And this gave me, for the first time, a sense of the transcendent power of the human mind, and the fact that it might be equipped to discover or decipher the deepest secrets of nature, to read the mind of God (pp 190–191).
Many scientists like me fondly recollect their enchantment as children with interactive displays and periodic tables at various museums and even attribute their decision to enter their profession to the experience. Sacks’ description of the Science Museum’s periodic table was one of the factors inspiring the creation of Fresno’s own periodic table mural [14–16].
In a book written to enable a broad audience of general nonmathematical readers to attain scientific literacy, Peter W. Atkins asks his readers to suppose that the periodic table is an actual landscape, and he presents it as a travel guide to an imaginary country in which the elements are various regions [17]. Similarly, according to Sacks,
On this second visit I found myself looking at the table in almost geographical terms, as a realm, a kingdom, with different territories and boundaries (p 191).
In his discussion of the periodic system Sacks does not neglect Mendeleev’s predecessors such as Johann Wolfgang Döbereiner [18–20], Alexandre Émile Béguyer de Chancourtois, John Alexander Reina Newlands, and others, who had tried to classify the elements into some sort of rational scheme.
With his inquiring, intuitive mind Sacks speculated about possibilities that were later found to be correct. He conceived that xenon and fluorine might react to form a compound (pp 202–203) long before Neil Bartlett’s preparation of Xe[PtF6] [21], and in wondering if transuranium elements could form a second rare earth series he anticipated Glenn T. Seaborg’s actinide hypothesis (pp 209–210) [22].
Uncle Tungsten has been universally praised [23, 24] in reviews from a variety of viewpoints in dozens of periodicals. It has even been examined philosophically [25]. I propose to concentrate on the experiments described by Sacks that chemical educators may wish to use as lecture demonstrations or to have their students carry out in laboratory classes in connection with discussions of Uncle Tungsten. The book should serve as a potent antidote to the prevalent epidemic of chemophobia and anti-science attitudes infecting our students and our entire society. During my adolescence, I carried out many of these same experiments, and I have published specific directions for performing them. In the following list I first give the pages of Sacks’ text on which they are mentioned, followed by references to the articles or books by others or me.
· Aluminothermal reduction (Thermit or Goldschmidt reaction) (pp 23, 43, and 84) [26–28].
· Oxidation of aluminum by removal of the Al2O3 coating with mercury (pp 38–39) [29].
· Reaction of sodium with water (pp 122–123) [30, 31].
· Ignition of a potassium chlorate-sugar mixture with sulfuric acid (Sacks uses potassium perchlorate) (pp 42 and 77) [32, 33].
· Limelight (p 48) [34, 35].
· Pouring vinegar on a piece of chalk (p 67) [36].
· Neutralization with red cabbage as an indicator (p 67) [37, 38].
· Supersaturated solution (p 67) [39, 40].
· Chemical garden (p 68) [41–43].
· Burning of sodium in chlorine (p 74) [44].
· Explosion of a hydrogen and oxygen mixture (p 77) [45].
· Explosion of nitrogen triiodide (p 77) [46, 47].
· Ammonium dichromate volcano (p 78) [48].
· Charring of sugar with sulfuric acid (p 78) [49, 50].
· Blue tetraamminecopper(II) (p 79) [51, 52].
· Oxidation states (p 80) [53, 54].
· Reaction between iron filings and sulfur (p 82) [55].
· Ignition of zinc and iodine by addition of water (p 83) [56].
· Esters and their odors (p 87) [49, 57, 58].
· Phosphorus (pp 223-226) [59–62].
In the course of reading Sacks’ book—on almost every page—I kept asking myself why he abandoned his intense love affair with chemistry and instead became a neurologist. At the very end he finally answered my question:
It was “understood,” by the time I was fourteen, that I was going to be a doctor; my parents were doctors, my brothers in medical school. My parents had been tolerant, even pleased, with my early interests in science, but now, they seemed to feel, the time for play was over (p 309).
But his conversion was more than a case of St. Paul’s,
When I was a child, I spake as a child, I understood as a child, I thought as a child; but when I became a man, I put away childish things [63].
Rather, it was akin to the explanation offered with increasing frequency by turncoats who have abandoned one political party for the other, namely, that it was the party not the person that had changed:
Did this mean that chemists of the future (if they existed) would never actually need to handle a chemical; might never see the colors of vanadium salts, never smell a hydrogen selenide, never admire the form of a crystal; might live in a colorless, scentless mathematical world? This, for me, seemed an awful prospect, for I, at least, needed to smell and touch and feel, to place myself, my senses, in the middle of the perceptual world. I had dreamed of being a chemist, but the chemistry that really stirred me was the lovingly detailed, naturalistic, descriptive chemistry of the nineteenth century, not the new chemistry of the quantum age. Chemistry, as I knew it, the chemistry I loved, was either finished or changing its character, advancing beyond me (or so I thought at the time). I felt I had come to the end of the road, the end of my road, at least, that I had taken my journey into chemistry as far as I could (pp 312–313).
As a member of the generation that was raised with chemistry sets before the fear of litigation rendered such sets anachronisms [5–7], I was enamored with what we affectionately called “slop-bucket chemistry” so I can certainly empathize with his view. However, Sacks is not entirely “lost” to chemistry:
The passion for chemistry, which I had thought dead at fourteen, has clearly survived, deep inside me, throughout the intervening years. Though my life has taken a different direction, I have followed the new discoveries in chemistry with excitement (p 316).
Perhaps we may expect more chemical gems like Uncle Tungsten from Sacks in the future.
References and Notes
1. Jacobs, M. A. Chemical Boyhood Remembered. Chem. Eng. News 2000 (January 10), 78 (2), 10–14.
2. Sacks, O. A Pocket Spectroscope. Chem. Heritage Winter 2001/2002, 19 (4), 9, 40–43.
3. It’s Elemental: Chemical & Engineering News celebrates the Periodic Table of the Elements on the magazine’s 80th anniversary. Chem. Eng. News 2003 (September 8), 81 (36), 27–190; contains 90 short articles by leading authorities.
4. Sacks, O. Uncle Tungsten: Memories of a Chemical Boyhood. CHED Newsletter Fall 2003, Abstract 13.
5. Kauffman, G. B. My First Chemistry Set. Today's Chemist 1989 (December), 2 (6), 14–15.
6. Fuller, M. E. Uncle Tungsten. J. Chem. Educ. 2003, 80, 878.
7. Tyler, J. The Chemcraft Story: The Legacy of Harold Porter; St. Johann Press: Haworth, NJ, 2003. For a review see Kauffman, G. B. Chem. Educator 2004, 9, 339–340; DOI 10.1333/s00897040833a.
8. Strathern, P. Mendeleyev’s Dream: The Quest for the Elements; Hamish Hamilton, Penguin Books, London, 2000; Thomas Dunne Books, St. Martin’s Press: New York, 2000. For a review see Kauffman, G. B. Chem. Educator 2003, 8 (1), 87–89; DOI 10.1333/s00897030661a.
9. Knight, D. M. Humphry Davy: Science and Power; Blackwell Publishers: Cambridge, MA, 1992. For a review see Kauffman, G. B. Chem. Eng. News 1993 (August 2), 71(31), 32–33.
10. Kauffman, G. B. Aus Meinem Leben: Adventures and Travels of a Chemical Educator-Historian-Researcher (George C. Pimentel Award in Chemical Education Acceptance Address). Chemistry Education 1995 (January–March), 11 (3), 5–17.
11. Fullmer, J. Z. Young Humphry Davy: The Making of an Experimental Chemist; American Philosophical Society: Philadelphia, PA, 2000. For a review see Kauffman, G. B. Chem. Educator 2002, 7 (6), 401–403; DOI 10.1333/s00897020644a.
12. Davy, H. The Collected Works of Sir Humphry Davy; Davy, J., Ed.; Introduction by Knight, D. M.; Thoemmes Press: Bristol, UK; Sterling, VA, 2001. For a review see Kauffman, G. B. Chem. Educator 2004, 9, 337–339; DOI 10.1333/s00897040832a.
13. Shapley, H. Of Stars and Men: The Human Response to an Expanding Universe; Beacon Press: Boston, MA, 1958; pp 38–39.
14. Kauffman, G. B. Fresno unveils its own mural of periodic tables. The Fresno Bee, August 30, 2003, p B9.
15. California State University, Fresno: College of Science and Mathematics: Periodic Table Artwork. http://129.8.12.93/ln_center/kiosk/pertable2.html (accessed Nov 2004). Student comments about their chosen elements and artwork are included.
16. Kauffman, G. B.; Frank, D. L. Periodic Table Mural: A Community-Based Participatory Art and Science Project. Chem. Educator 2004, 9, 46–51; DOI 10.1333/s00897040753a.
17. Atkins, P. W. The Periodic Kingdom: A Journey into the Land of the Chemical Elements; BasicBooks: New York, 1995. For a review see Kauffman, G. B. J. Chem. Educ. 1996, 73, A177.
18. Kauffman, G. B. From Triads to Catalysis: Johann Wolfgang Döbereiner (1780–1849) on the 150th Anniversary of His Death. Chem. Educator 1999, 4, 186–197; DOI 10.1333/s00897990326a.
19. Cassebaum, H.; Kauffman, G. B. The Periodic System of the Chemical Elements: The Search for Its Discoverer. Isis 1971, 62, 314–327.
20. Kauffman, G. B. American Forerunners of the Periodic Law. J. Chem. Educ. 1969, 46, 128–135.
21. Kauffman, G. B. Looking Back: The First Noble Gas Compound. Industrial Chemist 1987 (July), 8 (7), 32–33.
22. Kauffman, G. B. Beyond Uranium: Discovery of the first two transuranium elements 50 years ago added a new dimension to the periodic table. Chem. Eng. News 1990 (November 19), 68 (47), 18–23, 26–29.
23. The only mildly negative criticism seems to have come from Peter J. T. Morris of the National Museum of Science and Industry in London (the site of Sacks’ periodic table mural), who sent an email to the History of Chemistry list (chem-hist@listserv.ngate.uni-regensberg.de), which he thought might be a “good forum for a discussion on a tricky issue” regarding some of Sacks’ recollections in Uncle Tungsten (January 16, 2002). Morris was concerned that “most reviewers have not been historians and have taken it at face value.” He feared that
given Sacks’ stellar reputation and his powerful prose style, his book is going to give rise, willy nilly, to a new mythology. To take two examples I can address myself. He says that the Science Museum’s periodic table was at the top of the stairs [p 187—GBK], when in fact it was actually within the chemistry gallery. He describes it as being in “a dark cabinet” [p 187—GBK] when in fact contemporary photographs show a display with a white background either in a well-lit glass case or more likely, on display in the open air (incredible as this may seem today).
Several correspondents pointed out that “the events related took place a long time ago and the book is as much about how Sacks remembers them more than the actual events themselves.” Morris concluded that
I do feel that these misconceptions (let’s not call them errors) do matter, however, as readers will assume that they are correct and as a historian, I believe errors do matter whatever the source (January 17, 2002).
24. The only errors that I found were the misspellings of “vanadium” as “vandium” (periodic table, pp 192–193) and “Comptes Rendus” as “Comptes Rendu” (p 201); as well as the identification of Neil Bartlett as an American (He was born in Newcastle-upon-Tyne, England, p 203).
25. Laszlo, P. The Plays of Boys. Hyle 2002, 8, 55–67; http://www.hyle.org/journal/issues/8-1/rev_laszlo.htm (accessed Nov 2004).
26. Kauffman, G. B. Tested Demonstrations: A Modified Thermit Lecture Demonstration. J. Chem. Educ. 1981, 58, 802; Favorite Demonstration: The Thermit Reaction: A Dazzling Thermochemical Demonstration. J. Coll. Sci. Teaching 1997, 26, 286–287.
27. Thermite Reaction. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 85–89.
28. The Thermite Process. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 54–59.
29. Kauffman, G. B.; Adams, M. L. Aluminium—An Active Metal. Educ. Chem. 1990 (March), 27 (3), 36–39; Favorite Demonstration: The Atmospheric Oxidation of Aluminum. J. Coll. Sci. Teaching December 1990/January 1991, 20, 178–179.
30. Kauffman, G. B.; Jackson, J. D. Favorite Demonstration: Sodium—Water Reactions. J. Coll. Sci. Teaching 1985, 14, 432; Sodium-Water Reactions. CHEM 13 NEWS, 1994 (May), 231, 13.
31. Schrader, C.; Marek, L.; Offutt, M.; Lehrer, T.; Lewis, B.; Kimble, G. Sodium: A Spectacular Element; Flinn Scientific, Inc.: Batavia, IL, 1999. For a review of this videocassette see Kauffman, G. B.; Melendy, A. Chem. Educator 2002, 7, 190–191; DOI 10.10/1333/s00897020565a.
32. Kauffman, G. B.; Jackson, J. D.; Stringer, S. D. The Oxidation of Sucrose by Potassium Chlorate. Chem. Education 1989 (April-June), 5(4), 60.
33. Reaction of Potassium Chlorate and Sugar. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 79–80.
34. Kauffman, G. B.; Yen, K. S. Favorite Demonstration: Creating a Limelight in the Laboratory. J. Coll. Sci. Teaching 1991, 21, 54–56. See also Kauffman, G. B.; Pennington, S. D. Favorite Demonstration: Sunny Side Up! The Exothermic Slaking of Lime. J. Coll. Sci. Teaching 1992, 22, 66–68; and Kauffman, G. B. It can be done [Frying an egg by slaking lime, calcium oxide], The Fresno Bee, July 28, 2002, p B8.
35. Reaction of Calcium Oxide and Water (Slaking of Lime). In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 19–20.
36. Fizzing and Foaming: Reactions of Acids with Carbonates. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1989; Vol. 3, pp 96–99.
37. Acid–Base Indicators Extracted from Plants. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1989; Vol. 3, pp 50–57.
38. Plant Dyes as Universal Indicators. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 126–128.
39. Kauffman, G. B.; Hagopian, J.; Ebner, R. D. Favorite Demonstration: Exothermic Crystallization from a Supersaturated Solution. J. Coll. Sci. Teaching December 1985/January 1986, 15, 236.
40. Crystallization from Supersaturated Solutions of Sodium Acetate. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 27–29.
41. Kauffman, G. B.; Ferguson, C. A. Favorite Demonstration: A Silicate Garden. J. Coll. Sci. Teaching 1986, 15, 491.
42. Colorful Stalagmites: The Chemical Garden. Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1989; Vol. 3, pp 379–380.
43. A Chemical Garden. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 23–24.
44. Reaction of Sodium and Chlorine. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 61–63.
45. Explosive Reaction of Hydrogen and Oxygen. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 106–112.
46. Explosive Decomposition of Nitrogen Triiodide. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 96–98.
47. Nitrogen Triiodide. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 292–293.
48. Decomposition of ammonium dichromate. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 81–82.
49. Kauffman, G. B.; Pennington, S. D. Favorite Demonstration: Sulfuric Acid: King of Chemicals—History, Chemistry, and Some Demonstrations of H2SO4. J. Coll. Sci. Teaching 1993, 22, 383–386; reprinted in Favorite Demonstrations for College Science; Shmaefsky, B. R., Ed.; An NSTA Press Journals Collection, NSTA Press: Arlington, VA, 2004; pp. 109–114.
50. Dehydration of Sugar by Sulfuric Acid. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 77–78.
51. Kauffman, G. B.; Karbassi, M. Tested Demonstrations: An Improved Demonstration of the Cuprammonium Rayon Process. J. Chem. Educ. 1985, 62, 878.
52. Cuprammonium Rayon. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 247–248.
53. Kauffman, G. B. Experiments with Unusual Oxidation States. Educ. Chem. 1975, 12, 103–105; Kauffman, G. B.; Ebner, R. D. Colorful Redox Reactions. Sci. Teacher 1992 (May), 59 (5), 62–64.
54. The Many Colors of Vanadium. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 62–63.
55. Reaction of Iron and Sulfur. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 55–56.
56. Reaction of Zinc and Iodine. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 49–50.
57. Ethyl Acetate. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 306–307.
58. Esters as Natural Perfumes. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 308–310.
59. Spontaneous Combustion of White Phosphorus. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 74-76.
60. Air Oxidation of White Phosphorus. In Shakhashiri, B. Z. Chemical Demonstrations: A Handbook for Teachers of Chemistry; University of Wisconsin Press: Madison, WI, 1983; Vol. 1, pp 186–189.
61. White Phosphorus, a Very Dangerous Chemical. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 41–44.
62. The Chemoluminescence of Phosphorus. In Roesky, H. W.; Möckel, K. Chemical Curiosities: Spectacular Experiments and Inspired Quotes; Mitchell, T. N.; Russey, W. E., Translators; VCH Publishers: New York, 1996; pp 203–204.
63. I Corinthians, 13: 11.
George B. Kauffman
California State University, Fresno, georgek@csufresno.edu
S1430-4171(04)06852-3, 10.1333/s00897040852a
The Quotable Scientist: Words of Wisdom from Charles Darwin, Albert Einstein, Richard Feynman, Galileo, Marie Curie, and More. By Leslie Alan Horvitz. McGraw-Hill: New York, 2000. x + 169 pp, hardbound, 14.5 ´ 21.8 cm. $14.95. ISBN 0-07-136063-8.

Since the beginning of my academic career I’ve been an enthusiastic collector of quotations, bons mots, and aphorisms, both scientific and nonscientific, for use in my lectures, articles, and books. Year after year, whenever textbook salesmen visited my office in search of salable manuscripts, I repeatedly inquired about the marketability of a book of quotations—a kind of scientific Bartlett’s Familiar Quotations. Their response was consistently negative. However, such a venture must have since become financially feasible, for beginning in 1977 with British crystallographer Alan L. Mackay’s collection of such quotations [1], there have been a number of additional books of this type [2–5].
The Quotable Scientist is a volume in McGraw-Hill’s “Quotable” series, which includes The Quotable Writer by William A. Gordon, The Quotable Historian by Alan Axelrod, The Quotable Woman by Carol Turkington, and The Quotable Executive by John Woods. Its compiler, Leslie Alan Horvitz, is the author of several books, including Frontiers of Science, and is a frequent contributor to the Washington Times and Science Times.
The quotes are arranged in 46 short sections, some divided into subsections. The longest section is Scientific Method (71 quotations), and the shortest is Cloning (2 quotations). Sections probably of most interest to chemical educators and the number of quotations in them are Chemistry (9), Physics (49), Atom (9), and Molecules (2), but some quotations related to chemistry appear in other sections, for example, August Kekulé’s dream of the benzene ring appears in the section on Discoveries, Insights, and Epiphanies. The remaining sections are: The Practice and Purpose of Science, History of Science, Scientists, Inventions, Nature, Taxonomy, Evolution, Biology, Aging, Human Anatomy, The Brain, Consciousness, Medicine, Genetics, Animals, Ornithology, Entomology, Botany, Time, Climate, Earth and the Environment, Oceans, Mathematics and Science, Chaos, Geology, Quantum Physics, Nuclear Power, Astronomy, Cosmology, Extraterrestrial Life, Creation, Purpose of Creation, Chance and Necessity, Scientific Disputes, Risks and Limitations of Science, Predictions, Catastrophes, The Future, and Unsolved Mysteries.
According to Horvitz, who feels that the media has led the public to think that scientists are poor communicators,
While The Quotable Scientist is by no means capable of remedying the inevitable distortions of the media, it is in some way intended as a corrective to at least some misconceptions about how science is actually carried on. If it can give readers a taste of how scientists actually undertake their exploration—first in the lab (or in the field) and then on paper, as described in their own words—then it will have served its purpose (p ix).
While Horvitz has fulfilled his purpose in this collection, and his selections demonstrate wonder, curiosity, insight, and humor on the part of scientists, his book, which is eminently suitable for browsing, has a number of deficiencies. As the subtitle of the volume indicates, many of the quotations are from only a few persons (Darwin, Einstein, etc.). Furthermore, many of the recent quotations have been taken from several other books of interviews [6–8], which could be consulted directly. Although the book is relatively short, an index, which is usually provided in collections of this type, would have been useful. More serious is the failure to include reference citations, dates, or context for all of the quotations. In many cases only a name is given. Also, the hallmark of such a collection is accuracy, and misspellings of names, for example, “Sachs” for “Sacks” (Oliver), “Hutchenson” for “Hutchinson” (Clyde), “Monad” for Monod” (Jacques), “Shawlow” for “Schawlow” (Arthur), “Margeneau” for “Margenau” (Henry), “Rene” for “René” (Dubos), “Weizsacker” for “Weizsäcker” (Carl Friedrich von), and “Kekule” for “Kekulé” (August), cast doubt about the accuracy of the quotations themselves.
The collection includes many familiar quotations such as “The eternal mystery of the world is its comprehensibility.” (Albert Einstein), “It has not escaped our notice that the specific pairing that we have postulated immediately suggests a possible copying mechanism for the genetic material.” (James D. Watson and Francis H. C. Crick), and Asimov’s three laws of robotics, but there are many that were new to me such as “If the basic idea is too complicated to fit on a T-shirt, it’s probably wrong.” (Leon Lederman) and “The first principle is that you must not fool yourself, and you’re the easiest person to fool.” (Richard Feynman). Although the quotations are all related to science, the persons who are quoted are by no means limited to scientists; the selections include such diverse names as Hilaire Belloc, William Jefferson Clinton, Ecclesiastes, George III of Great Britain, Johann Wolfgang von Goethe, James Joyce, John Locke, Karl Marx, Henry David Thoreau, Arnold Toynbee, and the inevitable Yogi Berra (“Prediction is very hard, especially when it’s about the future.”).
This collection should be a valuable resource for practicing scientists, mathematicians, writers, philosophers, trivia buffs, speakers, lecturers, educators, and anyone for whom quotations are of interest, but better and more comprehensive books are available. However, because of its modest price and the fact that the above audience will not wish to depend on any one book, it may find a place in their personal libraries. Academic and public libraries will also wish to purchase it.
References and Notes
1. Mackay, A. L., Compiler; Scientific Quotations: The Harvest of a Quiet Eye; Crane, Russak & Co.: New York, 1977. For a review see Kauffman, G. B. Isis 1978, 69, 273–274. The collection later appeared in an expanded second edition with a different title and a different publisher: Mackay, A. L. Dictionary of Scientific Quotations; Institute of Physics Publishing: Bristol, England/Philadelphia, PA, 1991.
2. Albert Einstein, the first scientific super star, has been a fruitful source of quotations, and collections limited to his quotes are: Calaprice, A., Collector and Compiler; The Quotable Einstein; Princeton University Press: Princeton, NJ, 1996. For a review see Kauffman, G. B.; Kauffman, L. M. Am. Sci. 1998, 86, 82–83; and Calaprice, A., Collector and Compiler; The Expanded Quotable Einstein; Princeton University Press: Princeton, NJ, 2000. For a review see Kauffman, G. B.; Kauffman, L. M. Chem. Educator 2002, 7, 239–241; DOI 10.1333/s00897020586a.
3. Gaither, C. C.; Cavazos-Gaither, A. E., Selectors and Arrangers; Scientifically Speaking: A Dictionary of Quotations; Institute of Physics: Bristol, England/Philadelphia, PA, 2002. For a review see Kauffman, G. B. Chem. Educator 2002,7, 387; DOI 10.1333/s00897020631a. The Institute of Physics Publishing has contributed a number of inexpensive paperback volumes all by the same selectors and arrangers in this somewhat neglected genre, which I intend to review in the future: Statistically Speaking: A Dictionary of Quotations, 1996; Physically Speaking: A Dictionary of Quotations on Physics and Astronomy, 1997; Mathematically Speaking: A Dictionary of Quotations, 1998; Practically Speaking: A Dictionary of Quotations on Engineering, Technology and Architecture, 1998; Medically Speaking: A Dictionary of Quotations on Dentistry, Medicine and Nursing, 1999; Naturally Speaking: A Dictionary of Quotations on Biology, Botany, Nature and Zoology, 2000; Chemically Speaking: A Dictionary of Quotations, 2002.
4. Fripp, J.; Fripp, M.; Fripp, D. Compilers and Editors; Speaking of Science: Notable Quotes on Science, Engineering, and the Environment; LLH Technology Publishing: Eagle Rock, VA, 2000.
5. Kaplan, R. Science Says: A Collection of Quotations on the History, Meaning, and Practice of Science; A Stonesong Press Book, W. H. Freeman & Co.: Oxford/New York, 2001.
6. Hall, S. S. Mapping the Next Millennium: The Discovery of New Geographies; Vintage Books: New York, 1992.
7. Brian, D. Genius Talk: Conversations with Nobel Scientists and Other Luminaries; Kluwer Academic Press: Dordrecht, The Netherlands, 1995.
8. Kaku, M. Visions: How Science Will Revolutionize the 21st Century; Anchor Books, Doubleday: Garden City, NY, 1998.
George B. Kauffman
California State University, Fresno, georgek@csufresno.edu
S1430-4171(04)06853-2, 10.1333/s00897040853a
Super Vision: A New View of Nature. By Ivan Amato. Foreword by Philip Morrison. Harry N. Abrams, Inc.: New York, 2003. More than 200 Illustrations. 232 pp, hardbound, 24.2 ´ 28.6 cm. $40.00 (USA); $65.00 (Canada); £25.00. ISBN 0-8109-4545-4.

Front Cover. To weave its web a spider brews a concoction of liquid protein in its silk glands and pushes it through cylindrical spigots in its abdomen. Each of the strands shown here is about 3–5 microns (3-5 ´ 10–6 meter).
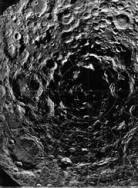
Back Cover. A composite of 1500 images from the Clementine mission maps the Moon’s south polar region in unprecedented scope and detail. The area shown spans about 1200 kilometers (1.2 ´ 106 meters).
For years I have maintained that research is as much an art as a science [1], that the scientist is as personally involved in his or her creation as is the artist, and that the sciences and humanities have much in common, The Two Cultures [2], notwithstanding.
Rather than one scientific method, there are myriad methods of science, and a scientist may choose and combine them to express his own individual personality much as an artist selects and blends his colors. Some scientists work with established classical techniques; others prefer to create new media. Some work with elaborate instruments so complex as to stagger the imagination; others choose to limit themselves to the simplest and most elementary tools. Some employ the language of higher mathematics; some produce results that are strictly qualitative. The work of some is theoretical and highly abstract; the work of others is eminently practical. The variations and the satisfactions are virtually endless. In his choice of a problem that he considers worthwhile and in his choice of the methods to solve it, the true scientist, as opposed to the technician who is content to repeat routines mechanically, exercises his individually. Who says that the scientist doesn’t make value judgments? [3].
In Fresno the CSUF College of Science and Mathematics has initiated several art projects to enhance the public spaces of the main Science Building, including an interactive Periodic Table of the Elements mural, in which the elements or their applications are depicted in artistic representations submitted by regional middle and high school students [4]. My claim that art and science are intimately related has been amply confirmed from various quarters. The theme for the American Chemical Society’s 2001 National Chemistry Week was “Celebrating Chemistry and Art” [5], and Hyle, the international journal for the philosophy of chemistry, devoted an entire issue to the relationship [6]. Competitions of scientific photographs as art have been held such as the Science and Engineering Visualization Challenge, cosponsored by the journal Science and the National Science Foundation [7] and the Small World Competition sponsored by Nikon, the camera and optical equipment firm [8].
Scientific images can be enhanced and rendered more artistic, intriguing, and surprising by using instruments to change the scale either in terms of size or time. A scientific book that takes advantage of the difference in scale is one of my favorites—Philip and Phylis Morrison’s Powers of Ten, the first volume in the Scientific American Library book club series [9], which consists of, among other exciting sections, 42 photographs ranging from a cosmic 1025 meters (about one billion light-years) to a subnuclear 10–16 meters (0.1 fermi). In addition to hardbound and paperbound editions, it is now available as a videocassette [10] as well as an interactive CD-ROM disk [11], which make ideal gifts for budding—or even mature—scientists. Other images that owe their fascination to differences in scale have appeared recently [12–14].
Now that sub-Ångström images—of a silicon crystal—have been achieved by electron microscopy at the Oak Ridge National Laboratory [15], we may expect further artistic as well as scientific resultsin this field. Harold E. Edgerton’s pioneering development of ultrafast photography provides typical examples of the use of speed or time in producing beautiful artistic images [16–18].
In his foreword to the book under review here, Philip Morrison, Institute Professor Emeritus and Professor Emeritus of Physics at the Massachusetts Institute of Technology and a member of the National Academy of Sciences, who is well known for his numerous books as well as films and television specials such as the PBS series “The Ring of Truth,” states,
Since the 1970s and 1980s, when my wife and I worked with the office of Charles and Ray Eames on both the film and book versions of Powers of Ten, an examination of the sizes of objects in the universe and their relative scale, scientific imagery has only become more varied and revealing. What’s more, it is increasingly blurring the lines between two Grand Categories, science and art (p 8).
Today scientific instruments and technologies, such as radio, X-ray, and Hubble space telescopes; light, polarizing, electron, atomic force, birefringence imaging, scanning probe, and scanning tunneling microscopes; microlithographs; gamma-ray and scanning gate spectroscopes; ultraviolet, visible, and infrared spectrometers; gravity detectors; X-ray diffraction cameras; computer-aided tomography (CT) scans; electroencephalograms; radar; particle accelerators; magnetic field and thermal detectors; magnetic resonance and hyperspectral overhead imagers; three-dimensional sonography systems; ion, fluorescence, and chemically mapping probes; computers; and neutron activation analysis, have greatly extended the visual range of the human eye and have enabled us to see much more of the physical world—from fundamental particles smaller than atomic nuclei to the incomprehensibly vast structure of the universe.
These devices can capture millions of colors invisible to the naked eye, look back in cosmic time to the extent of 14 billion years, peer behind and within apparently opaque barriers like skin and bone, and even catch events lasting no longer than a trillionth of a second. This image-driven capacity has provided us with what freelance print and radio science writer Ivan Amato calls Super Vision—a power “far more potent than Superman’s X-ray vision.”
Amato is Associate Editor of Science News, the recipient of the 1995 American Chemical Society’s James T. Grady-James H. Stack Award for Interpreting Chemistry for the Public and the 2001 Foresight Prize in Communication, and author of Stuff: The Materials the World Is Made Of, a New York Times Notable Book of 1997(BasicBooks: New York, 1997) and Pushing the Horizon (U.S. Government Printing Office: Washington, DC, 1998), an institutional history of the U.S. Naval Research Laboratory. He has also written for magazines such as Time, Fortune, U.S. News & World Report, Scientific American, Discover, Technology Review, and Wired, many newspapers, and National Public Radio.
Inspired by the beauty of the natural world as are the scientists who have produced the images, Amato has selected more than 200 breathtaking illustrations, including 170 plates in full color, depicting phenomena ranging across 42 orders of magnitude for this magnificent collection, which represents a comprehensive collection of the very best in scientific imagery. But this gorgeous oversize volume is much more than a beautiful coffee-table book. Each image is accompanied by a lively, detailed, accurate description of what is being viewed along with a lucid explanation of the underlying technology involved that includes definitions of pertinent technical terms and processes.
Amato explains his choices:
Each image that ultimately made it into this book moved me first on the æsthetic level….I searched for pictures whose colors, forms, and composition drew my eye and kept it there. Some colors were not present in the raw images recorded from, say, an electron microscope. Instead, using image-processing tools, they were added digitally by the scientists themselves or by others, with the goal of highlighting specific features of the images or making them more visually striking. I also strove to assemble a portfolio that illustrates the astounding powers of observation wielded by today’s armamentarium of scientific instruments. And I aimed to assemble a collection that would provide a pastiche of imagery reflective of the fantastically varied elements that mark the scientific way of telling stories about the world. Quite often, after learning about the meaning and context of a specific image, it would move me in a second way, one that combines an intellectual and an emotional component….The pictures also speak of new technologies that will transform how we live….If individual atoms have become our playthings, where can the line of technological impossibility be drawn? (p 17).
The volume begins with a one-page explanation of the scale of the universe, “The Orders of Magnitude: A Sense of the Whole Shebang” (p 9), followed by a six-page fold-out chart of the orders of magnitude encompassed in the book (10–15 meter to 1026 meters) with a different color for each of the ten ranges of orders of magnitude (pp 10–15). A two-page preface (pp 16–17) is followed by a 12-page introduction that presents a panorama of the human compulsion to record sensory experience from paleolithic cave paintings to the most radical abstract paintings and amounts to a short course in the history of science, instrumentation, and art (pp 18–29). The outer edge of each page of the main part of the book (pp 30–227) features a bar of the color corresponding to the particular range for that image to key it to its place in “the whole shebang.” The images are arranged in order of size from subatomic particles to galaxies of the universe. A three-page, triple-column index facilitates the location of persons, instruments, techniques, and images (pp 229–231).
Among the dazzling array of images to which we are treated are genes (p 34), a heartbeat (p 44), nerve cells (pp 50–51), chromosomes (p 53), trachea lining (p 56), twisted crystals (p 62), metal surfaces (p 65), hairs from a turtle’s ear (p 71), a guinea pig’s eye (p 114), snowflakes (among the few black and white images, pp 148–151), Intel Corporation’s Pentium 4 chip (p 158), fractal forms (pp 166–167), artificially induced electrical patterns (Lichtenberg patterns) (pp 172–173), the Sun’s vibrations (p 212), and cosmic background radiation (p 225).
Not all of nature is benign, as shown by images of the human immunodeficiency virus (HIV) (p 55) and a Bacillus anthracis cell (pp 66–67). Edgerton’s ultrafast photography is represented by a speeding bullet and a drop falling into a basin of water (pp 156–157). The similarity between nature and art is drawn by images of stained human brain cells that resemble a Jackson Pollack painting (pp 101–103), cancerous dog skin that is reminiscent of Vincent van Gogh’s “Starry Night” (pp 126–127), and a dog’s skin mole that is the “microscopic equivalent of a cubist painting” (p 127).
Super Vision is both a primer on the scientific worldview and a reminder of the awesome, multidimensional beauty of the natural world. Both informative and delightful, this unique balanced blend of science and art reveals much of what would be otherwise hidden in nature without the advanced, cutting-edge instrumentation featured here. The book itself, apart from the images that it contains, is a true work of art printed with wide margins on heavy, glossy paper. Although art books are notoriously expensive, it is an unbelievable bargain for a volume of this type, and it would make a beautiful gift for anyone with an interest in and appreciation for science and art. In short, I agree heartily with Oliver Sacks, who declared, “A whole history of science and technology, of seeing and imaging, is contained in this extraordinary book.
References and Notes
1. Kauffman, G. B. An Introduction to the Art of Scientific Research: A Personal View. The 1973 California State University and Colleges Outstanding Professor Award Lecture, October 25, 1973. In Teaching Excellence: A Collection of Essays on College Education Written by Recipients of the California State University Trustees’ Outstanding Professor Award; Flachmann, M., Ed.; California State University Institute: Long Beach, CA, 1998; pp 19–33; for an abbreviated version see A Personal View: Introduction to the Art of Scientific Research. J. Coll. Sci. Teaching 1973, 3, 124–129.
2. Snow, C. P. The Two Cultures and the Scientific Revolution; Cambridge University Press: New York, 1961.
3. Kauffman, G. B. Science and the Scientist—A Personal Interpretation. Fresno State College Alumni Journal 1967 (March), 7–9. I am surprised—and embarrassed—to find that at the time I did not consider that scientists could be women. If I were writing this passage today, I would use “he or she” and “his or her.” Mea maxima culpa.
4. Kauffman, G. B. Fresno unveils its own mural of periodic tables. The Fresno Bee, August 30, 2003, p B9; Kauffman, G. B.; Frank, D. L. Periodic Table Mural: A Community-Based Participatory Art and Science Project. Chem. Educator 2004, 9, 46–51; DOI 10.1333/s00897040753a.
5. Kauffman, G. B. Chemistry and History: Celebrating Chemistry and Art: National Chemistry Week 2001. Chem. Educator 2001, 6, 389–395; DOI 10.1333/s00897010523a; There is art in those mysterious chemicals. The Fresno Bee, November 3, 2001, p B7.
6. Special Issue on Æsthetics and Visualization in Art (A Virtual Art Exhibition on CD-ROM). Hyle 2003, 9, http://hyle.org/ (accessed Nov 2004). For a review see Fictorie, C. P. J. Chem. Educ. 2004, 81, 955–956.
7. Suplee, C.; Bradford, M. 2004 Visualization Challenge. Science 2004, 305, 1903–1907. See also Rovner, S. Communicating Science: Science as Art: Competition sponsored by Science and NSF honors beauteous art. Chem. Eng. News 2004 (September 27), 82 (39), 6 and A Visual Tour from the Heights of Mt. Etna to Tiny Antarctic Molecules. http://www.aaas.org/news/releases/2004/0923sevc.shtml (accessed Nov 2004).
8. Helmuth, L. Magnificent Magnifications: Microscope Jockeys from around the World Enter Their Masterpieces in an Annual Art Show. Smithsonian 2004 (October), 84–88.
9. Morrison, P.; Morrison, P. Powers of Ten: A Book about the Relative Size of Things in the Universe and the Effect of Adding Another Zero; Scientific American Books, W. H. Freeman: New York, 1982. For a review see Kauffman, G. B. J. Coll. Sci. Teaching December, 1984/January, 1985, 14, 213.
10. Powers of Ten: The Films of Charles and Ray Eames, Volume 1; Pyramid Film & Video: Santa Monica, CA, 1989. For a review of this videocassette see Kauffman, G. B.; Kauffman, L. M. J. Coll. Sci. Teaching 1994, 23, 381–382.
11. Powers of Ten Interactive; written, produced, and designed by Eames Demetrios; Eames Office: Venice, CA, 1999. For a review of this CD-ROM disk see Kauffman, G. B.; Zellmer, D. L. J. Coll. Sci. Teaching 2000, 29, 445.
12. Frankel, F. Sightings. Am. Sci. 2004, 92, 462–463.
13. Knoeber, R. “Do You See What I See? The Fresno Bee, August 30, 2004, p D1; August 31, 2004, pp E1, E3.
14. Goodsell, D. S. Bionanotechnology: Lessons from Nature; Wiley-Liss: Hoboken, NJ, 2004.
15. Nellist, P. D. et al. Direct Sub-Angstrom Imaging of a Crystal Lattice. Science 2004, 305, 1741. Also see Dagani, R. Surface Science: See Down to 0.6 Å: Electron microscope achieves direct sub-angstrom imaging of a crystal. Chem. Eng. News 2004 (September 20), 82 (38), 13.
16. Bruce, R. R., Ed. Seeing the Unseen: Dr. Harold E. Edgerton and the Wonders of Strobe Alley; MIT Press: Cambridge, MA, 1994.
17. Kayafas, G.; Jussim, E. Stopping Time: The Photographs of Harold Edgerton; Harry N. Abrams: New York, 2000.
18. Edgerton, H. E. Exploring the Art and Science of Stopping Time: A CD-ROM Based on the Life and Work of Harold E. Edgerton; MIT Press: Cambridge, MA, 2000.
George B. Kauffman
California State University, Fresno, georgek@csufresno.edu
S1430-4171(04)06854-1, 10.1333/s00897040854a
Handbook of Applied Surface and Colloid Chemistry. Krister Holmberg, Editor; Dinesh O. Shah and Milan J. Schwuger, Associate Editors. John Wiley & Sons, Ltd.: Chichester, England/New York, 2002. http://www.wiley.co.uk; http://www.wiley.com. 2 volumes, xxxiii + 1076 pp, hardcover, 19.5 ´ 25.3 cm. $795.00. ISBN 0-471-49083-0. Email for orders and customer service inquiries: cs-books@wiley.co.uk.

This book is the only work of its type in the field. Comprehensive, up-to-date books on the fundamentals of surface chemistry, such as Lyklema’s Fundamentals of Interface Colloid Science [1], are available, as are excellent books on surface chemistry in general, for example, Evans and Wennerström’s The Colloidal Domain [2] and Rosen’s Surfactants and Interfacial Phenomena [3]. However, the Handbook of Applied Surface and Colloid Chemistry is unique in dealing with applied surface chemistry in a broad sense.
This handbookis an international undertaking. Editor Krister Holmberg, Head of the Department of Applied Surface Chemistry at the Chalmers University of Technology, Göteborg, Sweden, who contributed two chapters, has had wide experience in various aspects of the field. He has worked in industry and has been Director of the internationally renowned Ytkemiska Institutet (the Institute for Surface Chemistry, misspelled “Yskemska” on p xiii) in Stockholm. Associate Editor Dinesh O. Shah, who contributed one chapter, works at the Center for Surface Science and Engineering, Department of Chemical Engineering, University of Florida, Gainesville (my alma mater), and Associate Editor Milan J. Schwuger works at the Forschungszentrum Jülich, Jülich, Germany. The 70 contributors from academic and industrial laboratories and research institutes hail from ten countries—Germany (20); Sweden (17); the United States (15, seven from the University of Florida); the UK (7); France and Canada (3 each); Italy (2); and Australia, Switzerland, and Ukraine (one each).
In order to limit the handbook to a reasonable length, the editors have restricted the coverage to “wet” as opposed to “dry” surface chemistry. Consequently, significant applications of dry surface chemistry, for example, heterogeneous catalysis involving gases and important vacuum analysis techniques, such as electronic spectroscopy for chemical analysis (ESCA) and selected-ion mass spectrometry (SIMS) are not considered. However, the most important applications, phenomena, and analytical techniques within the area of wet surface chemistry are included.
The Handbook of Applied Surface and Colloid Chemistry is replete with hundreds of tables, figures, mathematical equations, chemical formulas and equations, and eight color plates. Each of its two volumes, which are separately paginated (all pages are double-column format.), contains tables of contents for both volumes, a list of contributors and their addresses, a one-page foreword, a two-page preface, and three detailed indexes (for Volume 1, 11 pp; for Volume 2, 10 pp; and Cumulative, 19 pp). Each of its 45 overview chapters, which survey all the practical aspects of surface and colloid chemistry, is provided with an up-to-date bibliography of references, ranging in length from five citations (Volume 2, Chapter 22) to 237 citations (Volume 2, Chapter 20). The references include books, articles (with full titles), and patents; some are as recent as 2001, and a few were still in press at the time of publication of the book.
Each of the chapters is written as a separate entity and can be read independently. In the words of Brian Vincent, who wrote the foreword,
An attractive feature of this book is that the author of each chapter has been given the freedom to present, as he/she sees fit, the spectrum of the relevant science, from pure to applied, in his/her particular topic. Of course this approach inevitably leads to some overlap and repetition in different chapters, but this does not necessarily matter. This arrangement should be extremely useful to the reader (even if it makes the book look longer), since one does not have to search around in different chapters for various bits of related information. Furthermore, any author will naturally have his own views on, and approach to, a specific topic….It is often useful for someone else, particularly a newcomer, wanting to research a particular topic, to have different approaches presented to them [sic] (p xiii).
The handbook is divided into five parts. The chapters in Part 1 deal with a broad range of industrial and household uses—pharmaceuticals, food, detergency, agriculture, photography, paints, paper-making, emulsion polymerization, ceramics processing, mineral processing, and oil production. Part 2 discusses the four major classes of surfactants (anionics, nonionics, cationics, and zwitterionics), polymeric and novel surfactants, hydrotropes, and other topics. Part 3 discusses, among other subjects, the four colloidal systems—solid dispersions (suspensions), foams, vesicles and liposomes, and microemulsions. Holmberg admits that a chapter on emulsions should have been added here, but it was never written. However, Chapter 8 of Volume 1 provides a thorough general treatment of emulsions, while Chapter 1 of Volume 2 gives a good background to colloidal stability, which is relevant to emulsions. Part 4 reviews the important phenomena of foam breaking, solubilization, rheological effects of surfactants, wetting, spreading, and penetration. Part 5 concerns a selected number of important experimental techniques. Although most books related to analysis and characterization are arranged into chapters on different methods, the division here is according to problem. A list of the chapters and their lengths shows the wide range of topics to which the fundamental science has been applied:
Volume 1 (xvi + 485 pp)
Part 1. “Surface Chemistry in Important Technologies”
Chapter 1. “Surface Chemistry in Pharmacy” (38 pp)
Chapter 2. “Surface Chemistry in Food and Feed” (14 pp)
Chapter 3. “Surface Chemistry in Detergency” (21 pp)
Chapter 4. “Surface Chemistry in Agriculture” (11 pp)
Chapter 5. “Surface and Colloid Chemistry in Photographic Technology” (20 pp)
Chapter 6. “Surface Chemistry in Paints” (18 pp)
Chapter 7. “Surface Chemistry of Paper” (51 pp, the handbook’s longest chapter)
Chapter 8. “Surface Chemistry in the Polymerization of Emulsion” (26 pp)
Chapter 9. “Colloidal Processing of Ceramics” (18 pp)
Chapter 10. “Surface Chemistry in Dispersion, Flocculation and Flotation” (31 pp)
Chapter 11. “Surface Chemistry in the Petroleum Industry” (17 pp)
Part 2. “Surfactants”
Chapter 12. “Anionic Surfactants” (22 pp)
Chapter 13. “Nonionic Surfactants” (14 pp)
Chapter 14. “Cationic Surfactants” (40 pp)
Chapter 15. “Zwitterionic and Amphoteric Surfactants” (24 pp)
Chapter 16. “Polymeric Surfactants” (12 pp)
Chapter 17. “Speciality Surfactants” (21 pp)
Chapter 18. “Hydrotropes” (14 pp)
Chapter 19. “Physico-Chemical Properties” (23 pp)
Chapter 20. “Surfactant-Polymer Systems” (19 pp)
Chapter 21. “Surfactant Liquid Crystals” (44 pp)
Chapter 22. “Environmental Aspects of Surfactants” (28 pp)
Chapter 23. “Molecular Dynamics Computer Simulations of Surfactants” (14 pp)
Volume 2 (xvi + 591 pp)
Part 3. “Colloidal Systems and Layer Structures at Surfaces”
Chapter 1. “Solid Dispersions” (21 pp)
Chapter 2. “Foams and Foaming” (21 pp)
Chapter 3. “Vesicles” (9 pp)
Chapter 4. “Microemulsions” (23 pp)
Chapter 5. “Langmuir-Blodgett Films” (20 pp)
Chapter 6. “Self-Assembling Monolayers: Alkane Thiols on Gold” (18 pp)
Part 4. “Phenomena in Surface Chemistry”
Chapter 7. “Wetting, Spreading and Penetration” (24 pp)
Chapter 8. “Foam Breaking in Aqueous Systems” (15 pp)
Chapter 9. “Solubilization” (30 pp)
Chapter 10. “Rheological Effects in Surfactant Phases” (26 pp)
Part 5. “Analysis and Characterization in Surface Chemistry”
Chapter 11. “Measuring Equilibrium Surface Tensions” (8 pp, the shortest chapter)
Chapter 12. “Measuring Dynamic Surface Tensions” (14 pp)
Chapter 13. “Determining Critical Micelle Concentration” (11 pp)
Chapter 14. “Measuring Contact Angle” (30 pp)
Chapter 15. “Measuring Micelle Size and Shape” (17 pp)
Chapter 16. “Identification of Lyotropic Liquid Crystalline Mesophases” (34 pp)
Chapter 17. “Characterization of Microemulsion Structure” (34 pp)
Chapter 18. “Measuring Particle Size by Light Scattering” (14 pp)
Chapter 19. “Measurement of Electrokinetic Phenomena in Surface Chemistry” (12 pp)
Chapter 20. “Measuring Interactions between Surfaces” (32 pp)
Chapter 21. “Measuring the Forces and Stability of Thin-Liquid Films” (19 pp)
Chapter 22. “Measuring Adsorption” (10 pp)
According to editor Holmberg,
Taken together, the chapters constitute a an enormous wealth of surface and colloid chemistry knowledge and the book should be regarded as a rich source of information, arranged in a way that I hope the reader will find useful (p xv).
I agree with his opinion, and I am happy to recommend the Handbook of Applied Surface and Colloid Chemistry, an up-to-date comprehensive reference source, to materials scientists in both industry and academia as well as to persons working in a wide range of technologies concerned with colloid and surfactant research, development, and manufacture. Because it is practically rather than theoretically oriented, it should also complement existing textbooks on surface and colloid science, which generally take the more traditional approach of systematically reviewing the fundamental aspects of the subject, while adding a few examples of applications by way of illustration. This definitive handbook should also find a place in every academic, industrial, or technical library.
References and Notes
1. Lyklema, J., Ed. Fundamentals of Interface and Colloid Science; Academic Press: San Diego, CA; Vol. I. Fundamentals, 2nd ed., 1993; Vol. II. Solid-Liquid Interfaces, 1995; Vol. III. Liquid–Liquid Interfaces, 2000; Vols. IV and V in preparation.
64. Evans. D. F.; Wennerström, H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet, 2nd ed.; John Wiley & Sons: Hoboken, NJ, 1999.
65. Rosen, M. J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley-Interscience: Hoboken, NJ, 2004.
George B. Kauffman
California State University, Fresno, georgek@csufresno.edu
S1430-4171(04)06855-0, 10.1333/s00897040855a
Cold Fusion: Fire from Water: A Documentary about One of the Greatest and Most Controversial Scientific Discoveries of All Time. Written by Eugene F. Mallove and Jed Rothwell; narrated by James “Scotty” Doohan; produced and directed by Christopher Toussaint. A Free Spirit Production; Cold Fusion Technology, Inc.: Concord, NH, 1999. 68-min VHS videocassette. $29.95; To order call (603) 228-4516 or email staff@infinite-energy.com; FAX: (603) 485-4710. Infinite Energy Magazine, P.O. Box 2816, Concord, NH 03302-2816; web site: http://www.infinite-energy.com. ISBN 1-892925-01-X

On March 23, 1989, in a press conference at the University of Utah, chemistry professors B. Stanley Pons of the University of Utah and Martin Fleischmann, F.R.S. of the University of Southampton, England announced that they had achieved nuclear fusion by passing an electric current through a platinum wire anode coiled around a palladium cathode in a solution of alkaline heavy water (2H2O). Hailed as a source of unlimited energy, this alleged discovery of cold or room-temperature nuclear fusion (Actually, Pons and Fleischmann never used the term “cold fusion.”)was without a doubt the most publicized—and most controversial—scientific event of 1989.
From its unusual beginning, with a press conference announcement in place of the customary peer-reviewed article published in a scholarly journal, the cold fusion controversy involved to an unprecedented degree the interaction of science, politics, and the mass media. The world scientific community was polarized into two opposing camps—believers and skeptics. One of us (GBK) chronicled the controversy for five successive years in his “Applied Chemistry” feature in the Encyclopædia Britannica’s Yearbook of Science and the Future [1], by which time it appeared that Pons and Fleischmann’s claims had not been confirmed.
The media had a field day with the story. To an unusual extent articles appeared in newspapers and popular magazines, with Time, Newsweek, and Business Week devoting cover stories to the controversy. By early 1990 full-length books in various languages on the controversy had already appeared, and additional books soon followed [2–5]. Scientists in laboratories throughout the world obtained conflicting results (which one journalist characterized as “Elvis sightings”) in their attempts to detect the heat, neutrons, and tritium (3H) cited as evidence that fusion was actually occurring. In a New York Times Magazine article Nicholas P. Samos and Robert P. Crease, the Director and Historian, respectively, of Brookhaven National Laboratory, Upton, NY, called the cold fusion confusion “pathological science,” a term first used by 1932 Nobel chemistry laureate Irving Langmuir to characterize the kind of “discovery” made by outsiders (chemists instead of physicists in this case) who claim miraculous results and who respond with ad hoc excuses for the inability of others to replicate their results.
During succeeding years work continued in laboratories throughout the world, and annual International Conferences on Cold Fusion were held on what might prove to be “the scientific breakthrough of the century” or a self-deluded will-of-the–wisp like René Blondlot’s “N-rays” or a misinterpretation like Boris Deryagin’s “polywater.” At a time when antiscientific prejudice has increased, unfortunately, many people undoubtedly regarded the situation as further evidence of the shortcomings of science. Nevertheless, the controversy, with all its ambiguity, can serve as a case study of the manner in which science progresses and of the provisional nature of scientific “truth” and was already being used as such in undergraduate science courses. Regardless of the final outcome, it is certain to find a place in the history and sociology of science.
In The Mysterious Island (1875) Jules Verne predicted, “I believe that one day we will light and heat our homes with water…” The impressive, provocative, and visually breathtaking documentary videocassette, Cold Fusion: Fire From Water, released by the proponents of cold fusion on the 10th anniversary of Pons and Fleischmann’s announcement and which has aired on several PBS stations and in Europe, deals with just this subject—energy from water. We are treating it here in the spirit of “Audiatur et altera pars” (Let the other side also be heard).
Cold fusion promises that a great deal of energy can be generated simply from water and a small amount of input energy, allowing the world to move beyond the petrochemical age in which we currently live. According to this video, the study of the theory of cold fusion and attempts to bring it to fruition were unfortunately dealt a serious setback in credibility when Pons and Fleischmann came forward with their cold fusion claims, which Fleischmann, in this video, retrospectively admits may have been premature. Incidentally, less than twelve hours before Pons and Fleischmann’s announcement the Exxon Valdez ran aground in Alaska, resulting in one of the world’s worst environmental disasters. The video claims that these two events occurred so closely together as to be more than just coincidental.
The documentary, which won the 1999 Aurora Award Platinum Best of Show and 2000 21st Annual Telly Award and was a finalist for the 2000 Silver Axiem Award, is narrated by James “Scotty” Doohan of “Star Trek” fame. It begins with the Pons and Fleischmann incident and the extensive political and credibility fallout that followed. The U.S. Congress even held hearings to determine what really happened, and the U.S. Department of Energy published a report, which, according to some, “killed” the subject of cold fusion. Furthermore, several other highly respected laboratories performed experiments to confirm or disprove Pons and Fleischmann’s results.
One of these was a laboratory at the Massachusetts Institute of Technology, where, in addition to performing experimental work, the scientists also held a mock funeral of sorts for cold fusion. However, according to this video, after more careful analysis, it appears as though these scientists may have fudged or falsified the data that they ultimately presented in their paper. Eugene F. Mallove, later Editor of Infinite Energy: The Magazine of New Energy Technology, “a bimonthly publication designed for both general and technical readers,” and the video’s coauthor, resigned his position of chief science writer for the MIT News Office in protest over the manner in which the research results had been presented. Unfortunately, Dr. Mallove (1947–2004) was murdered on May 14, 2004 in Norwich, CT, where he was cleaning up a recently vacated property, a home in which he spent much of his childhood.
After allowing the major critics of cold fusion to speak, the documentary goes on to present scientists at a number of other laboratories, who discuss their positive results, admittedly achieved not every time but in anywhere from one out of ten to two out of three attempts. All of the scientists, technologists, and engineers are clearly identified. Positive results were claimed by Texas A & M electrochemist and Distinguished Professor John O’Mara Bockris, some of whose colleagues declared that his research on cold fusion was equivalent to misconduct. They unsuccessfully attempted to have his tenure revoked or at least that he be censured. However, according to the video program, he ultimately persisted in his efforts and triumphed, as did many other scientists. Positive results have been attained elsewhere, for example, at the Los Alamos National Laboratory, the University of Illinois, the Toyota Corporation, EPRI (Electric Power Research Institute, which is funded by the utility companies), and SRI International.
Some small start-up companies that are carrying out cold fusion research, such as Black Light Power, are obtaining patents for novel forms of cold fusion-type electrochemical cells, for which the controversial term “cold fusion” is never used. Equally as amazing are the results achieved by individual scientists working alone, such as those by Dr. Les Case, whose work has exerted a huge impact on the direction of cold fusion research. It seems very clear that something novel is occurring in these experiments, not every time the experiment is conducted, but as one scientist put it, “If positive results occur only once, the inquisitive scientist should ask why—a question that must be answered.”
The documentary does a good job in illustrating the political nature of research funding here in the United States and how the scientific establishment looks upon research that does not follow its strictly preconceived notions. Futurist, engineer, and author Sir Arthur C. Clark discusses the case of the Wright brothers and the total disregard with which the establishment of their day regarded their claims of airplane flight. Contemporary newspapers did not even mention the Wright brothers’ test flights for about five years. Sir Arthur, who was originally skeptical but is now 99% convinced, suggests that quite possibly cold fusion is in its infancy and that the world is on the cusp of a major revolution. The ramifications of successful, reliable cold fusion would be larger than the industrial and computer revolution combined. In his words,
It could be the end of the fossil fuel age: the end of oil and coal. And the end, incidentally, of many of our worries about global pollution and global warming.
Regarding the disbelief of establishment scientists, he says,
When a distinguished but elderly scientist states that something is possible, he is almost certainly right. When he states that something is impossible, he is very probably wrong.
And as Sir Arthur and others believe, cold fusion development will probably occur by private sector commercialization, thus totally bypassing the scientific establishment. The documentary emphasizes that in the past sometimes the greatest discoveries and contributions have encountered resistance from the mainstream scientific establishment because of challenges to accepted scientific notions. (The cases of radioactivity and transmutation of elements by nuclear reaction are cited.)
Mallove summarizes the present status of cold fusion research and boldly predicts its future:
Today we can no longer say that the evidence is overwhelmingly compelling. It is now 100% certain….Commercial activities are under way to develop this new scientific phenomenon, which we certainly still do not fully understand. I believe the phenomenon will be developed irrespective of whether we understand it….But at the same time…the scientific establishment appears absolutely deaf to all of this evidence. So I conclude that the only way that cold fusion will be triumphant will be in the commerciaIization. I think the electric power grid will absolutely wither away. I think automobiles, trucks, trains, planes—all forms of transportation—will use this new, powerful energy source, whatever its exact nature turns out to be. The handwriting is on the wall. The fossil fuel age is about to end.
However, we must close this review with a caution to the viewer and chemical educator that this video, which provides much food for thought and will be useful for courses in the sociology and history of science, should be supplemented with material reflecting the opposing viewpoint.
References and Notes
1. Kauffman, G. B. “Applied Chemistry: Cold Fusion,” in Yearbook of Science and the Future; Encyclopædia Britannica: Chicago, IL, 1991; pp 302–304; 1992, pp 302–303; 1993, pp 305–306; 1994, pp 308–310; 1995, pp 307–308.
2. Close, F. Too Hot to Handle: The Race for Cold Fusion; Princeton University Press: Princeton, NJ, 1991.
3. Mallove, E. F. Fire from Ice: Searching for the Truth Behind the Cold Fusion Furor; Infinite Energy Press: Concord, NH, 1991.
4. Huizenga, J. R. Cold Fusion: The Scientific Fiasco of the Century; University of Rochester Press: Rochester, NY, 1993.
5. Taubes, G. Bad Science: The Short Life and Weird Times of Cold Fusion; Random House: New York, NY, 1993.
George B. Kauffman and Hiram William Blanken
California State University, Fresno,
georgek@csufresno.edu, bmblanken02@comcast.net
S1430-4171(04)06856-X, 10.1333/s00897040856a
Encyclopedia of the Elements—Technical Data, History, Processing, Applications, 1st edition. By Per Enghag. Wiley-VCH: New York, 2004. 1309 pp, hardcover, 9.5 ´ 7.2 ´ 2.4 in. 259 €, $320.00. ISBN: 3-527-30666-8.
The chemical elements are the chemist’s “dollar,” the basic currency unit of the trade. The one-hundred-plus chemical elements known to date are being dealt with in a systematic fashion in textbooks of inorganic chemistry. The Encyclopedia of the Elements takes a novel approach to introduce chemists and other chemically oriented scientists to the basic building blocks of the chemical world.
The book—at more than twelve-hundred pages it qualifies as a tome—is subdivided into fifty-two chapters. A casual glance at the periodic table reveals that this is not the number of chemical elements known. A number of elements have received combined treatment in group chapters. The most notable of these “distillations” are the lanthanide (4f) elements with their unsurpassed degrees of similarity that led to many of them being named -dym (from dymos, greek for “twin”). The concluding chapter is devoted to “the radioactive elements,” a rather loose, chemically heterogenous bunch. The chapter preceding it deals with the noble gases.
All elements—whether they have a chapter of their own or whether they share one with some of their siblings—get the same basic treatment. The element in question is briefly introduced and chemical as well as physical parameters are given. These data, including a short paragraph on chemical characterization, come in a neatly arranged format, most of the numerical data in well-designed tables. All data are presented in a very readable, easy-to-use way, not crammed into tight paragraphs as is the case in many dictionaries and encyclopedias. After the “technical” sections, which will be cherished by scientists and engineers alike, there follow text-oriented sections on the discovery of the respective element(s), its/their use, function in biological systems (if any), and related matters including health and environmental issues.
The textual sections vary considerably in length, reflecting either the amount of knowledge gathered, or—more frequently—the importance from a human point of view of the element. The chapter on silicon, for example, is one of the most extensive, rivaled by those on carbon, iron and a few others. Elements of lesser importance (such as the heavier alkali metals) tend to be grouped into single chapters. The grouping does not apply to the introductory, technical parts, though, which are separately given for every element.
The author does have a special, easily detectable interest in the history of the discovery of the chemical elements. In the sections on this topic, the text usually switches to “narrative mode”, and Per Enghag introduces many intriguing stories related to how the elements were discovered (or indeed synthesized, as is the case for most of the trans-uranium super-heavy elements).
These essays on how the elements have been found and characterized frequently make fascinating reading, for this information is not normally available (at least not in this level of detail) in textbooks of inorganic chemistry. They span the full range from nonscientific alchemy to modern hard science, from unintended chance discoveries to systematic, targeted element hunting.
In the sections on manufacture and use, the discussion reaches beyond the realm of chemistry, touching on neighboring disciplines, such as mineralogy and mining, materials science, modern technology, and biology. Despite this broad approach, all parts are easy to read and understand with a moderate general scientific background. While much of the tabulated technical data will be only meaningful and useful for the professional, most of the remainder—in particular the text sections—will be of interest and can be read even by upper-level pupils in secondary school.
The Encyclopedia of the Elements is, therefore, of interest to a broad range of users, from pupils preparing for examinations or writing essays for science classes to the professional scientist in academic and industrial settings seeking specific data quickly.
The book is well researched and very accurate, providing the reader with reliable information. While it was not possible to check every entered number, of course, the encyclopedia seems to be as flawless as can be. The only minute discrepancy I came across is the statement on iridium that “Its density of 22.56 g/cm3 is the highest of all elements” (p 724), while the density of osmium, in agreement with other sources, is said to be higher: 22.60 g/cm3 (p 721). This is only a very minor mistake, however, in a book that by any measure is a most impressive piece of single-author work.
So is it full marks for this volume? Well, almost, give it eight-and-a-half out of ten. The one odd factor about it that provokes a comment is the arbitrary ordering of the entries. The arrangement of the entries does not follow any recognizable logic. The chemical elements are neither arranged in alphabetical order (which would be the straightforward simple principle one would normally expect from an encyclopedia!) nor are they arranged according to their grouping in the periodic table (which is the usual basis of organizing textbooks of inorganic chemistry, so scientists would be readily familiar with this principle). Although the Encyclopedia of the Elements does contain a very detailed table of contents that greatly facilitates easy navigation and localization of specific information, later editions will profit from a reshuffled organization built on either lexical or chemical logic.
An additional, minor point of criticism is the generous waste of printing space at the expense of readability due to a very small font size. Even if it would look a little bit less “sleek,” increasing the font size by two points would make more efficient use of the available page size and vastly increase the eye-friendliness of this wonderful piece of work. After all, this type of book is meant to be read rather than to behold!
A final and again minor suggestion for improvement would be the introduction of a periodic table inside the front or back cover of the book (even though most users will be familiar with the “chemical map of the world,” it is a well-established custom to have one in most textbooks of chemistry). In the age of digitized manuscripts and computer-assisted layout, these proposed changes amount to not more than a few day’s work. Except for a small number of entries in which more than one element is discussed in the same chapter (e.g. potassium/sodium; rubidium/cesium), shuffling the entries into alphabetical order could be done with the press of only a few keys.
These slight criticisms, which are mostly on the “cosmetic” side, do not at all diminish the usefulness of this first edition, though. The Encyclopedia of the Elements is a highly interesting addition to the chemical literature. It exhibits a distinguishable, even unique, character. I do not know of any other book that could at this moment be listed as a serious competitor. The chemical elements have been dealt with before by other authors, notably Peter Atkins and John Emsley. Their books, though, take a distinctly different, more popular, stance. The Encyclopedia of the Elements is a full-sized academic source of reference that will probably rapidly find lots of friends in all fields from (of course) inorganic chemistry, mineralogy, and materials science but probably among solid-state physicists and geochemists, too.
Unfortunately the price of this volume is way beyond the normal price range for a similar-sized textbook so institutions are likely to be the most significant market. It is to be hoped that it will have the success it deserves and sales will be such that the price (at least for later editions) may eventually be lowered to bring it into the realm of individual scientists’ purses (anorexic as they on average are). In the meantime, try to persuade your boss or the head of your library to get a copy.
Thomas Lazar
Göttingen (Germany), tlazar@web.de
S1430-4171(04)06857-9, 10.1333/s00897040857a